The fate of the primary cilium during myofibroblast transition
- PMID: 24403605
- PMCID: PMC3937090
- DOI: 10.1091/mbc.E13-07-0429
The fate of the primary cilium during myofibroblast transition
Abstract
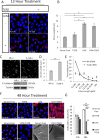
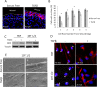
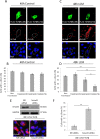
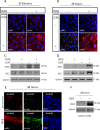
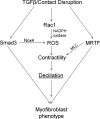
Myofibroblasts, the culprit of organ fibrosis, can originate from mesenchymal and epithelial precursors through fibroblast-myofibroblast and epithelial-myofibroblast transition (EMyT). Because certain ciliopathies are associated with fibrogenesis, we sought to explore the fate and potential role of the primary cilium during myofibroblast formation. Here we show that myofibroblast transition from either precursor results in the loss of the primary cilium. During EMyT, initial cilium growth is followed by complete deciliation. Both EMyT and cilium loss require two-hit conditions: disassembly/absence of intercellular contacts and transforming growth factor-β1 (TGFβ) exposure. Loss of E-cadherin-dependent junctions induces cilium elongation, whereas both stimuli are needed for deciliation. Accordingly, in a scratch-wounded epithelium, TGFβ provokes cilium loss exclusively along the wound edge. Increased contractility, a key myofibroblast feature, is necessary and sufficient for deciliation, since constitutively active RhoA, Rac1, or myosin triggers, and down-regulation of myosin or myocardin-related transcription factor prevents, this process. Sustained myosin phosphorylation and consequent deciliation are mediated by a Smad3-, Rac1-, and reactive oxygen species-dependent process. Transitioned myofibroblasts exhibit impaired responsiveness to platelet-derived growth factor-AA and sonic hedgehog, two cilium-associated stimuli. Although the cilium is lost during EMyT, its initial presence contributes to the transition. Thus myofibroblasts represent a unique cilium-less entity with profoundly reprogrammed cilium-related signaling.
Figures

Similar articles
-
β-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition.Mol Biol Cell. 2011 Dec;22(23):4472-85. doi: 10.1091/mbc.E11-04-0335. Epub 2011 Sep 30. Mol Biol Cell. 2011. PMID: 21965288 Free PMC article.
-
Differential topical susceptibility to TGFβ in intact and injured regions of the epithelium: key role in myofibroblast transition.Mol Biol Cell. 2013 Nov;24(21):3326-36. doi: 10.1091/mbc.E13-04-0220. Epub 2013 Sep 4. Mol Biol Cell. 2013. PMID: 24006486 Free PMC article.
-
Coordinate activities of BRD4 and CDK9 in the transcriptional elongation complex are required for TGFβ-induced Nox4 expression and myofibroblast transdifferentiation.Cell Death Dis. 2017 Feb 9;8(2):e2606. doi: 10.1038/cddis.2016.434. Cell Death Dis. 2017. PMID: 28182006 Free PMC article.
-
Smaddening complexity: the role of Smad3 in epithelial-myofibroblast transition.Cells Tissues Organs. 2011;193(1-2):41-52. doi: 10.1159/000320180. Epub 2010 Nov 3. Cells Tissues Organs. 2011. PMID: 21051861 Review.
-
LIM-domain proteins in transforming growth factor β-induced epithelial-to-mesenchymal transition and myofibroblast differentiation.Cell Signal. 2012 Apr;24(4):819-25. doi: 10.1016/j.cellsig.2011.12.004. Epub 2011 Dec 11. Cell Signal. 2012. PMID: 22182513 Review.
Cited by
-
Heterogeneity of the NIH3T3 Fibroblast Cell Line.Cells. 2022 Aug 28;11(17):2677. doi: 10.3390/cells11172677. Cells. 2022. PMID: 36078083 Free PMC article.
-
Primary cilia suppress the fibrotic activity of atrial fibroblasts from patients with atrial fibrillation in vitro.Sci Rep. 2024 May 30;14(1):12470. doi: 10.1038/s41598-024-60298-x. Sci Rep. 2024. PMID: 38816374 Free PMC article.
-
Insights into the Regulation of Ciliary Disassembly.Cells. 2021 Nov 1;10(11):2977. doi: 10.3390/cells10112977. Cells. 2021. PMID: 34831200 Free PMC article. Review.
-
Mechanism of ciliary disassembly.Cell Mol Life Sci. 2016 May;73(9):1787-802. doi: 10.1007/s00018-016-2148-7. Epub 2016 Feb 11. Cell Mol Life Sci. 2016. PMID: 26869233 Free PMC article. Review.
-
Mutations in the Kinesin-2 Motor KIF3B Cause an Autosomal-Dominant Ciliopathy.Am J Hum Genet. 2020 Jun 4;106(6):893-904. doi: 10.1016/j.ajhg.2020.04.005. Epub 2020 May 7. Am J Hum Genet. 2020. PMID: 32386558 Free PMC article.
References
-
- Barnes EA, Heidtman KJ, Donoghue DJ. Constitutive activation of the shh-ptc1 pathway by a patched1 mutation identified in BCC. Oncogene. 2005;24:902–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

