Development and characterization of a gastroretentive dosage form composed of chitosan and hydroxyethyl cellulose for alendronate
- PMID: 24403821
- PMCID: PMC3883582
- DOI: 10.2147/DDDT.S52791
Development and characterization of a gastroretentive dosage form composed of chitosan and hydroxyethyl cellulose for alendronate
Abstract
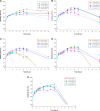
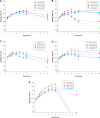
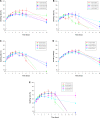
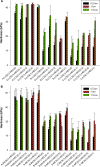
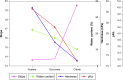
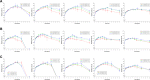
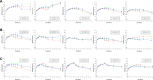
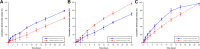
In this study, alendronate, the most commonly used biphosphonate for treating osteoporosis, was formulated as gastroretentive dosage form (GRDF) tablets to enhance its oral bioavailability. GRDF tablets were characterized with the effects of different molecular weights (MWs) of chitosan (CS) and hydroxyethyl cellulose (HEC) at various ratios on swelling, floating, and physical integrity. The CS component was formed using various acids: acetic, lactic, malic, succinic, and citric, and a high viscosity grade of HEC was selected. The results demonstrated that the swelling ratios of the formulations comprising high MW CS were lower than those of low or medium MW CS when salts were formed with any countering acids except for acetic acid. The decreasing ranking of the swelling rates was: CS-citrate > CS-malate > CS-lactate > CS-succinate > CS-acetate. A negative correlation was found between the pKa of the respective countering acid and the swelling rate. The swelling rate was promoted if an acidic salt of CS with a lower water content was incorporated, while it became slower when tablet hardness was higher or the compression force to form tablets was increased. Although HEC did not contribute to swelling or floating, it played a role in maintaining structural integrity. A prolonged dissolution profile of alendronate GRDF tablets developed in this study was observed.
Keywords: alendronate; chitosan; gastroretentive dosage form; hydrogel; hydroxyethyl cellulose; swelling.
Figures

References
-
- Streubel A, Siepmann J, Bodmeier R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr Opin Pharmacol. 2006;6(5):501–508. - PubMed
-
- Nayak AK, Maji R, Das B. Gastroretentive drug delivery systems: a review. Asian Journal of Pharmaceutical and Clinical Research. 2010;3(1):2–10.
-
- Hoffman A. Pharmacodynamic aspects of sustained release preparations. Adv Drug Deliv Rev. 1998;33(3):185–199. - PubMed
-
- Chawla G, Gupta P, Koradia V, Bansal AK. A means to address regional variability in intestinal drug absorption. Pharmaceutical Technology. 2003;27(7):50–68.
-
- Waterman KC. A critical review of gastric retentive controlled drug delivery. Pharm Dev Technol. 2007;12(1):1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

