Efficacy of Alfacalcidol on PEG-IFN/ Ribavirin Combination Therapy for Elderly Patients With Chronic Hepatitis C: A Pilot Study
- PMID: 24403915
- PMCID: PMC3877658
- DOI: 10.5812/hepatmon.14872
Efficacy of Alfacalcidol on PEG-IFN/ Ribavirin Combination Therapy for Elderly Patients With Chronic Hepatitis C: A Pilot Study
Abstract
Background: Serum vitamin D concentration is reported to show a decrease in older age. Patients with chronic hepatitis C (CHC) in Japan are older on average than those in Western countries. Moreover, the outcome of pegylated-interferon (PEG-IFN)/ ribavirin therapy combined with vitamin D in elderly patients is unclear.
Objectives: This pilot study explored the efficacy and safety of alfacalcidol as vitamin D source in PEG-IFN/ ribavirin combination therapy for elderly CHC patients infected with hepatitis C virus genotype 1b.
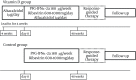
Patients and methods: Consecutive twenty CHC patients aged ≥ 65 years were enrolled in this pilot study. Fifteen patients met the inclusion criteria and received PEG-IFN/ ribavirin therapy combined with alfacalcidol. Four-week lead-in of oral alfacalcidol was conducted, and it was subsequently and concurrently administered in PEG-IFN/ ribavirin combination therapy (vitamin D group). Age, gender, and IL28B genotype-matched patients, who received PEG-IFN/ ribavirin alone, were saved as control group (n = 15) to compare the treatment outcome with the vitamin D group.
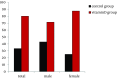
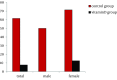
Results: Subjects consisted of 14 males and 16 females, with a median age of 70 years (65-78). The serum 25 (OH) D3 concentration in females (20 ng/ml, 11-37) was significantly lower than males (27 ng/mL, 13-49) (P = 0.004). Sustained virological response (SVR) rates were 33.3% (5/15) in the control group and 80.0% (12/15) in the vitamin D group, respectively (P = 0.025). While no significant difference was shown in the (SVR) rate between the two groups among males (P = 0.592), in females the SVR rate was significantly higher in the vitamin D group (87.5%, 7/8) than the control group (25.0%, 2/8) (P = 0.041). The relapse rates in the groups with and without alfacalcidol were 7.7% (1/13) and 61.5% (8/13), respectively (P = 0.011). Interestingly, in females, the relapse in the control group was shown in 5 of 7 (71.4%), whereas in the vitamin D group the relapse rate was decreased (1/8, 12.5%) (P = 0.041). No specific adverse events were observed in the vitamin D group.
Conclusions: PEG-IFN/ ribavirin combined with alfacalcidol may be effective and safe in elderly CHC patients. In particular, concomitant administration of alfacalcidol may lead to a reduced relapse rate, and consequently improving the SVR rate in elderly females.
Keywords: 1-hydroxycholecalciferol; Aged; Hepatitis C, Chronic; Ribavirin; Vitamin D.
Figures
Similar articles
-
Randomized study comparing vitamin D3 and 1α-Hydroxyvitamin D3 in combination with pegylated interferon/ribavirin therapy for chronic hepatitis C.J Gastroenterol Hepatol. 2015 Sep;30(9):1384-90. doi: 10.1111/jgh.12949. J Gastroenterol Hepatol. 2015. PMID: 25778685 Clinical Trial.
-
Virological response in patients with hepatitis C virus genotype 1b and a high viral load: impact of peginterferon-alpha-2a plus ribavirin dose reductions and host-related factors.Clin Drug Investig. 2008;28(1):9-16. doi: 10.2165/00044011-200828010-00002. Clin Drug Investig. 2008. PMID: 18081356
-
Effect of native vitamin D3 supplementation on refractory chronic hepatitis C patients in simeprevir with pegylated interferon/ribavirin.Hepatol Res. 2016 Mar;46(5):450-8. doi: 10.1111/hepr.12575. Epub 2015 Sep 17. Hepatol Res. 2016. PMID: 26289410
-
Treatment of chronic hepatitis C in southern Taiwan.Intervirology. 2006;49(1-2):99-106. doi: 10.1159/000087271. Intervirology. 2006. PMID: 16166797 Review.
-
[Possibilities of using cepeginterferon alpha-2b in double (cepeginterferon alfa-2b and ribavirin) and triple (simeprevir, cepeginterferon alpha-2b, and ribavirin) antiviral therapy regimens for chronic hepatitis C. A review of clinical trials and experience of everyday clinical practice].Ter Arkh. 2016;88(11):156-162. doi: 10.17116/terarkh20168811156-162. Ter Arkh. 2016. PMID: 28635836 Review. Russian.
Cited by
-
Vitamin D supplementation for chronic liver diseases in adults.Cochrane Database Syst Rev. 2017 Nov 3;11(11):CD011564. doi: 10.1002/14651858.CD011564.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Aug 25;8:CD011564. doi: 10.1002/14651858.CD011564.pub3. PMID: 29099543 Free PMC article. Updated.
-
Correlation between vitamin D levels and apoptosis in geriatric patients infected with hepatitis C virus genotype 4.Clin Interv Aging. 2016 May 4;11:523-33. doi: 10.2147/CIA.S104599. eCollection 2016. Clin Interv Aging. 2016. PMID: 27217734 Free PMC article.
-
Influencing factors on serum 25-hydroxyvitamin D3 levels in Japanese chronic hepatitis C patients.BMC Infect Dis. 2015 Aug 19;15:344. doi: 10.1186/s12879-015-1020-y. BMC Infect Dis. 2015. PMID: 26286329 Free PMC article.
-
Vitamin D and chronic hepatitis C: effects on success rate and prevention of side effects associated with pegylated interferon-α and ribavirin.Int J Clin Exp Med. 2015 Jul 15;8(7):10284-303. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26379820 Free PMC article. Review.
-
Vitamin D supplementation for chronic liver diseases in adults.Cochrane Database Syst Rev. 2021 Aug 25;8(8):CD011564. doi: 10.1002/14651858.CD011564.pub3. Cochrane Database Syst Rev. 2021. PMID: 34431511 Free PMC article.
References
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
