Moderate Recurrent Hypoglycemia Markedly Impairs Set-Shifting Ability in a Rodent Model: Cognitive and Neurochemical Effects
- PMID: 24403983
- PMCID: PMC3882127
- DOI: 10.2174/1876524601205010001
Moderate Recurrent Hypoglycemia Markedly Impairs Set-Shifting Ability in a Rodent Model: Cognitive and Neurochemical Effects
Abstract
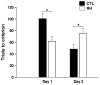
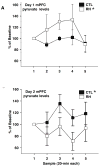
Recurrent hypoglycemia (RH) is the major complication of intensive insulin treatment for diabetes mellitus. Of particular concern is the perceived potential for long-term impact of RH on cognition. Because diabetic patients have been reported to have deficits in mental flexibility and judgment, both generally considered to be mediated predominantly by the prefrontal cortex, the purpose of the present study was to determine whether RH would affect prefrontal cortex function. Medial prefrontal cortex (mPFC)-mediated set-shifting ability was tested in male Sprague-Dawley rats using a maze-based, food-reward Set-Shift task analogous to the Wisconsin card-sorting task. The performance measure was the number of trials to criterion on both day 1 (initial rule-learning) and day 2 (set-shifting in response to a changed contingency). In vivo microdialysis was used to measure mPFC extracellular glucose, lactate, pyruvate, glutamate, and dopamine. Post-mortem measures within the mPFC included glucose transporter 3 (GluT3) and c-Fos. RH animals had enhanced performance on day 1, consistent with previous work that showed RH to improve subsequent hippocampal function when euglycemic. The key finding of the present work is that RH led to impaired set-shifting performance on day 2, suggesting impairment in e.g. mental flexibility. Consistent with this finding, RH animals show decreased mPFC glycolysis on day 2 compared to controls. Our data show that RH can lead to subsequent impaired judgment, accompanied by reduced prefrontal cortex function. The findings suggest a potential underlying mechanism for the impaired judgment seen in diabetic patients.
Keywords: Dopamine; glucose; medial prefrontal cortex; recurrent hypoglycemia; set-shifting ability.
Conflict of interest statement
None declared.
Figures





Similar articles
-
6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine.J Neurosci. 1994 May;14(5 Pt 1):2531-44. doi: 10.1523/JNEUROSCI.14-05-02531.1994. J Neurosci. 1994. PMID: 8182426 Free PMC article.
-
Bidirectional Optogenetically-Induced Plasticity of Evoked Responses in the Rat Medial Prefrontal Cortex Can Impair or Enhance Cognitive Set-Shifting.eNeuro. 2020 Jan 6;7(1):ENEURO.0363-19.2019. doi: 10.1523/ENEURO.0363-19.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31852759 Free PMC article.
-
Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats.Diabetes. 2004 Feb;53(2):418-25. doi: 10.2337/diabetes.53.2.418. Diabetes. 2004. PMID: 14747293
-
Neurochemical modulation of stress-induced cognitive inflexibility in a rat model of an attentional set-shifting task.Pharmacol Rep. 2013;65(6):1479-88. doi: 10.1016/s1734-1140(13)71508-1. Pharmacol Rep. 2013. PMID: 24552995 Review.
-
Reward-dependent learning in neuronal networks for planning and decision making.Prog Brain Res. 2000;126:217-29. doi: 10.1016/S0079-6123(00)26016-0. Prog Brain Res. 2000. PMID: 11105649 Review.
Cited by
-
Monocarboxylate Transporter 1 in the Medial Prefrontal Cortex Developmentally Expresses in Oligodendrocytes and Associates with Neuronal Amounts.Mol Neurobiol. 2017 Apr;54(3):2315-2326. doi: 10.1007/s12035-016-9820-7. Epub 2016 Mar 9. Mol Neurobiol. 2017. PMID: 26957300
-
GluT4: A central player in hippocampal memory and brain insulin resistance.Exp Neurol. 2020 Jan;323:113076. doi: 10.1016/j.expneurol.2019.113076. Epub 2019 Oct 12. Exp Neurol. 2020. PMID: 31614121 Free PMC article. Review.
-
Recurrent Moderate Hypoglycemia Suppresses Brain-Derived Neurotrophic Factor Expression in the Prefrontal Cortex and Impairs Sensorimotor Gating in the Posthypoglycemic Period in Young Rats.Dev Neurosci. 2016;38(1):74-82. doi: 10.1159/000442878. Epub 2016 Jan 28. Dev Neurosci. 2016. PMID: 26820887 Free PMC article.
-
Recurrent Hypoglycemia Increases Anxiety and Amygdala Norepinephrine Release During Subsequent Hypoglycemia.Front Endocrinol (Lausanne). 2015 Nov 20;6:175. doi: 10.3389/fendo.2015.00175. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26635724 Free PMC article.
-
Mental Work Requires Physical Energy: Self-Control Is Neither Exception nor Exceptional.Front Psychol. 2018 Jul 5;9:1005. doi: 10.3389/fpsyg.2018.01005. eCollection 2018. Front Psychol. 2018. PMID: 30026710 Free PMC article. Review.
References
-
- Spain M, Edlund BJ. Introducing insulin into diabetes management: transition strategies for older adults. J Gerontol Nurs. 2011;37(4):10–5. - PubMed
-
- Mudaliar S, Edelman SV. Insulin therapy in type 2 diabetes. Endocrinol Metab Clin North Am. 2001;30(4):935–82. - PubMed
-
- The Diabetes Control and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. - PubMed
-
- Tamborlane WV, Ahern J. Implications and results of the diabetes control and complications trial. Pediatr Clin North Am. 1997;44(2):285–300. - PubMed
-
- The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications Trial. Diabetes. 1997;46(2):271–86. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
