Enrichment of live unlabelled cardiomyocytes from heterogeneous cell populations using manipulation of cell settling velocity by magnetic field
- PMID: 24404002
- PMCID: PMC3585821
- DOI: 10.1063/1.4791649
Enrichment of live unlabelled cardiomyocytes from heterogeneous cell populations using manipulation of cell settling velocity by magnetic field
Abstract
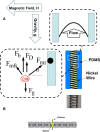
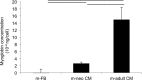
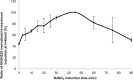
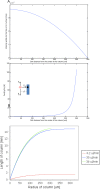
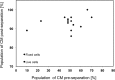
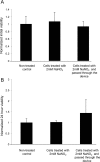
The majority of available cardiomyocyte markers are intercellular proteins, limiting our ability to enrich live cardiomyocytes from heterogeneous cell preparations in the absence of genetic labeling. Here, we describe enrichment of live cardiomyocytes from the hearts of adult mice in a label-free microfluidic approach. The separation device consisted of a vertical column (15 mm long, 700 μm diameter), placed between permanent magnets resulting in a field strength of 1.23 T. To concentrate the field at the column wall, the column was wrapped with 69 μm diameter nickel wire. Before passing the cells through the column, the cardiomyocytes in the cell suspension had been rendered paramagnetic by treatment of the adult mouse heart cell preparation with sodium nitrite (2.5 mM) for 20 min on ice. The cell suspension was loaded into the vertical column from the top and upon settling, the non-myocytes were removed by the upward flow from the column. The cardiomyocytes were then collected from the column by applying a higher flow rate (144 μl/min). We found that by applying a separation flow rate of 4.2 μl/min in the first step, we can enrich live adult cardiomyocytes to 93% ± 2% in a label-free manner. The cardiomyocytes maintained viability immediately after separation and upon 24 h in culture.
Figures

Similar articles
-
Size-based microfluidic enrichment of neonatal rat cardiac cell populations.Biomed Microdevices. 2006 Sep;8(3):231-7. doi: 10.1007/s10544-006-8169-5. Biomed Microdevices. 2006. PMID: 16732418
-
Label-free enrichment of functional cardiomyocytes using microfluidic deterministic lateral flow displacement.PLoS One. 2012;7(5):e37619. doi: 10.1371/journal.pone.0037619. Epub 2012 May 29. PLoS One. 2012. PMID: 22666372 Free PMC article.
-
[In vitro study of effects of transient receptor potential vanilloid 1 on autophagy in early hypoxic mouse cardiomyocytes and the mechanism].Zhonghua Shao Shang Za Zhi. 2019 Mar 20;35(3):186-192. doi: 10.3760/cma.j.issn.1009-2587.2019.03.005. Zhonghua Shao Shang Za Zhi. 2019. PMID: 30897864 Chinese.
-
Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury.Circulation. 2017 Aug 29;136(9):834-848. doi: 10.1161/CIRCULATIONAHA.116.024307. Epub 2017 Jun 22. Circulation. 2017. PMID: 28642276 Free PMC article.
-
Embryonic stem cell transplantation: promise and progress in the treatment of heart disease.BioDrugs. 2008;22(6):361-74. doi: 10.2165/0063030-200822060-00003. BioDrugs. 2008. PMID: 18998754 Review.
Cited by
-
Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells.Theranostics. 2017 May 17;7(7):2067-2077. doi: 10.7150/thno.19427. eCollection 2017. Theranostics. 2017. PMID: 28638487 Free PMC article. Review.
-
Fundamentals and application of magnetic particles in cell isolation and enrichment: a review.Rep Prog Phys. 2015 Jan;78(1):016601. doi: 10.1088/0034-4885/78/1/016601. Epub 2014 Dec 4. Rep Prog Phys. 2015. PMID: 25471081 Free PMC article. Review.
-
Microfluidic Sample Preparation for Single Cell Analysis.Anal Chem. 2016 Jan 5;88(1):354-80. doi: 10.1021/acs.analchem.5b04077. Epub 2015 Dec 3. Anal Chem. 2016. PMID: 26567589 Free PMC article. Review. No abstract available.
-
Microfluidic immunomagnetic cell separation using integrated permanent micromagnets.Biomicrofluidics. 2013 Oct 15;7(5):54115. doi: 10.1063/1.4825395. eCollection 2013. Biomicrofluidics. 2013. PMID: 24396526 Free PMC article.
-
Isolation of cells for selective treatment and analysis using a magnetic microfluidic chip.Biomicrofluidics. 2014 Jun 16;8(3):034114. doi: 10.1063/1.4883855. eCollection 2014 May. Biomicrofluidics. 2014. PMID: 25379074 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
