Development of vertical SU-8 microtubes integrated with dissolvable tips for transdermal drug delivery
- PMID: 24404018
- PMCID: PMC3625238
- DOI: 10.1063/1.4798471
Development of vertical SU-8 microtubes integrated with dissolvable tips for transdermal drug delivery
Abstract
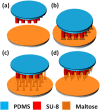
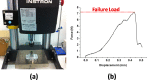
Polymer-based microneedles have drawn much attention in the transdermal drug delivery resulting from their flexibility and biocompatibility. Traditional fabrication approach deploys various kinds of molds to create sharp tips at the end of needles for the penetration purpose. This approach is usually time-consuming and expensive. In this study, we developed an innovative fabrication process to make biocompatible SU-8 microtubes integrated with biodissolvable maltose tips as novel microneedles for the transdermal drug delivery applications. These microneedles can easily penetrate the skin's outer barrier represented by the stratum corneum (SC) layer. The drug delivery device of mironeedles array with 1000 μm spacing between adjacent microneedles is proven to be able to penetrate porcine cadaver skins successfully. The maximum loading force on the individual microneedle can be as large as 7.36 ± 0.48N. After 9 min of the penetration, all the maltose tips are dissolved in the tissue. Drugs can be further delivered via these open biocompatible SU-8 microtubes in a continuous flow manner. The permeation patterns caused by the solution containing Rhodamine 110 at different depths from skin surface were characterized via a confocal microscope. It shows successful implementation of the microneedle function for fabricated devices.
Figures







Similar articles
-
Development of vertical SU-8 microneedles for transdermal drug delivery by double drawing lithography technology.Biomicrofluidics. 2013 Dec 6;7(6):66501. doi: 10.1063/1.4843475. eCollection 2013. Biomicrofluidics. 2013. PMID: 24396551 Free PMC article.
-
Fabrication, characterization and application of sugar microneedles for transdermal drug delivery.Ther Deliv. 2017 Mar;8(5):249-264. doi: 10.4155/tde-2016-0096. Ther Deliv. 2017. PMID: 28361607
-
Fiber optic microneedles for transdermal light delivery: ex vivo porcine skin penetration experiments.J Biomech Eng. 2010 Sep;132(9):091014. doi: 10.1115/1.4002192. J Biomech Eng. 2010. PMID: 20815648
-
Current trends in polymer microneedle for transdermal drug delivery.Int J Pharm. 2020 Sep 25;587:119673. doi: 10.1016/j.ijpharm.2020.119673. Epub 2020 Jul 30. Int J Pharm. 2020. PMID: 32739388 Free PMC article. Review.
-
Microneedles: A versatile strategy for transdermal delivery of biological molecules.Int J Biol Macromol. 2018 Apr 15;110:30-38. doi: 10.1016/j.ijbiomac.2017.12.027. Epub 2017 Dec 6. Int J Biol Macromol. 2018. PMID: 29223756 Review.
Cited by
-
Microneedle-Based Glucose Sensor Platform: From Vitro to Wearable Point-of-Care Testing Systems.Biosensors (Basel). 2022 Aug 6;12(8):606. doi: 10.3390/bios12080606. Biosensors (Basel). 2022. PMID: 36005002 Free PMC article. Review.
-
Microarray patches enable the development of skin-targeted vaccines against COVID-19.Adv Drug Deliv Rev. 2021 Apr;171:164-186. doi: 10.1016/j.addr.2021.01.022. Epub 2021 Feb 2. Adv Drug Deliv Rev. 2021. PMID: 33539853 Free PMC article. Review.
-
Development of vertical SU-8 microneedles for transdermal drug delivery by double drawing lithography technology.Biomicrofluidics. 2013 Dec 6;7(6):66501. doi: 10.1063/1.4843475. eCollection 2013. Biomicrofluidics. 2013. PMID: 24396551 Free PMC article.
-
Dissolving Microneedle Patches for Dermal Vaccination.Pharm Res. 2017 Nov;34(11):2223-2240. doi: 10.1007/s11095-017-2223-2. Epub 2017 Jul 17. Pharm Res. 2017. PMID: 28718050 Free PMC article. Review.
-
Toward Self-Powered Wearable Adhesive Skin Patch with Bendable Microneedle Array for Transdermal Drug Delivery.Adv Sci (Weinh). 2016 Apr 19;3(9):1500441. doi: 10.1002/advs.201500441. eCollection 2016 Sep. Adv Sci (Weinh). 2016. PMID: 27711262 Free PMC article.
References
-
- Nir Y., Paz A., Sabo E., and Potasman I., Am. J. Trop. Med. Hyg. 68, 341 (2003). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
