Guillain-Barré syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: a multinational self-controlled case series in Europe
- PMID: 24404128
- PMCID: PMC3880265
- DOI: 10.1371/journal.pone.0082222
Guillain-Barré syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: a multinational self-controlled case series in Europe
Abstract
Background: The risk of Guillain-Barré syndrome (GBS) following the United States' 1976 swine flu vaccination campaign in the USA led to enhanced active surveillance during the pandemic influenza (A(H1N1)pdm09) immunization campaign. This study aimed to estimate the risk of GBS following influenza A(H1N1)pdm09 vaccination.
Methods: A self-controlled case series (SCCS) analysis was performed in Denmark, Finland, France, Netherlands, Norway, Sweden, and the United Kingdom. Information was collected according to a common protocol and standardised procedures. Cases classified at levels 1-4a of the Brighton Collaboration case definition were included. The risk window was 42 days starting the day after vaccination. Conditional Poisson regression and pooled random effects models estimated adjusted relative incidences (RI). Pseudo likelihood and vaccinated-only methods addressed the potential contraindication for vaccination following GBS.
Results: Three hundred and three (303) GBS and Miller Fisher syndrome cases were included. Ninety-nine (99) were exposed to A(H1N1)pdm09 vaccination, which was most frequently adjuvanted (Pandemrix and Focetria). The unadjusted pooled RI for A(H1N1)pdm09 vaccination and GBS was 3.5 (95% Confidence Interval (CI): 2.2-5.5), based on all countries. This lowered to 2.0 (95% CI: 1.2-3.1) after adjustment for calendartime and to 1.9 (95% CI: 1.1-3.2) when we accounted for contra-indications. In a subset (Netherlands, Norway, and United Kingdom) we further adjusted for other confounders and there the RI decreased from 1.7 (adjusted for calendar month) to 1.4 (95% CI: 0.7-2.8), which is the main finding.
Conclusion: This study illustrates the potential of conducting European collaborative vaccine safety studies. The main, fully adjusted analysis, showed that the RI of GBS was not significantly elevated after influenza A(H1N1)pdm09 vaccination (RI = 1.4 (95% CI: 0.7-2.8). Based on the upper limits of the pooled estimate we can rule out with 95% certainty that the number of excess GBS cases after influenza A(H1N1)pdm09 vaccination would be more than 3 per million vaccinated.
Conflict of interest statement
Figures

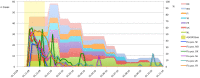
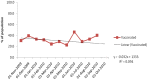
References
-
- Lu CY, Shao PL, Chang LY, Huang YC, Chiu CH, et al. (2010) Immunogenicity and safety of a monovalent vaccine for the 2009 pandemic influenza virus A (H1N1) in children and adolescents. Vaccine 28: 5864–5870. - PubMed
-
- Lu W, Tambyah PA (2010) Safety and immunogenicity of influenza A H1N1 vaccines. Expert Rev Vaccines 9: 365–369. - PubMed
-
- Wijnans L, de Bie S, Dieleman J, Bonhoeffer J, Sturkenboom M (2011) Safety of pandemic H1N1 vaccines in children and adolescents. Vaccine 29: 7559–7571. - PubMed
-
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, et al. (1979) Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol 110: 105–123. - PubMed
-
- Hurwitz ES, Schonberger LB, Nelson DB, Holman RC (1981) Guillain-Barré syndrome and the 1978–1979 influenza vaccine. N Engl J Med 304: 1557–1561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

