Pediatric obesity and vitamin D deficiency: a proteomic approach identifies multimeric adiponectin as a key link between these conditions
- PMID: 24404137
- PMCID: PMC3880269
- DOI: 10.1371/journal.pone.0083685
Pediatric obesity and vitamin D deficiency: a proteomic approach identifies multimeric adiponectin as a key link between these conditions
Abstract
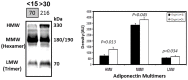
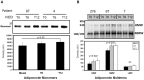
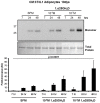
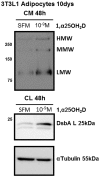
Key circulating molecules that link vitamin D (VD) to pediatric obesity and its co-morbidities remain unclear. Using a proteomic approach, our objective was to identify key molecules in obese children dichotomized according to 25OH-vitamin D (25OHD) levels. A total of 42 obese children (M/F = 18/24) were divided according to their 25OHD3 levels into 25OHD3 deficient (VDD; n = 18; 25OHD<15 ng/ml) or normal subjects (NVD; n = 24; >30 ng/ml). Plasma proteomic analyses by two dimensional (2D)-electrophoresis were performed at baseline in all subjects. VDD subjects underwent a 12mo treatment with 3000 IU vitamin D3 once a week to confirm the proteomic analyses. The proteomic analyses identified 53 "spots" that differed between VDD and NVD (p<0.05), amongst which adiponectin was identified. Adiponectin was selected for confirmational studies due to its tight association with obesity and diabetes mellitus. Western Immunoblot (WIB) analyses of 2D-gels demonstrated a downregulation of adiponectin in VDD subjects, which was confirmed in the plasma from VDD with respect to NVD subjects (p<0.035) and increased following 12mo vitamin D3 supplementation in VDD subjects (p<0.02). High molecular weight (HMW) adiponectin, a surrogate indicator of insulin sensitivity, was significantly lower in VDD subjects (p<0.02) and improved with vitamin D3 supplementation (p<0.042). A direct effect in vitro of 1α,25-(OH)2D3 on adipocyte adiponectin synthesis was demonstrated, with adiponectin and its multimeric forms upregulated, even at low pharmacological doses (10(-9) M) of 1α,25-(OH)2D3. This upregulation was paralleled by the adiponectin interactive protein, DsbA-L, suggesting that the VD regulation of adiponectin involves post-transciptional events. Using a proteomic approach, multimeric adiponectin has been identified as a key plasma protein that links VDD to pediatric obesity.
Conflict of interest statement
Figures


References
-
- Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266–281. - PubMed
-
- Martini LA, Wood RJ (2006) Vitamin D status and the metabolic syndrome. Nutr Rev 64: 479–486. - PubMed
-
- Song Q, Sergeev IN (2012) Calcium and vitamin D in obesity. Nutr Res Rev 16: 1–12. - PubMed
-
- Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, et al. (2013) Maternal and newborn vitamin D status and its impact on food allergy development in the german LINA cohort study. Eur J Allergy Clin Immunol 68: 220–228. - PubMed
-
- Ford ES, Ajani UA, McGuire LC, Liu S (2005) Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 28: 1228–1230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

