Human mammospheres secrete hormone-regulated active extracellular vesicles
- PMID: 24404144
- PMCID: PMC3880284
- DOI: 10.1371/journal.pone.0083955
Human mammospheres secrete hormone-regulated active extracellular vesicles
Abstract
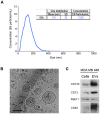
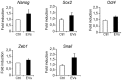
Breast cancer is a leading cause of cancer-associated death worldwide. One of the most important prognostic factors for survival is the early detection of the disease. Recent studies indicate that extracellular vesicles may provide diagnostic information for cancer management. We demonstrate the secretion of extracellular vesicles by primary breast epithelial cells enriched for stem/progenitor cells cultured as mammospheres, in non-adherent conditions. Using a proteomic approach we identified proteins contained in these vesicles whose expression is affected by hormonal changes in the cellular environment. In addition, we showed that these vesicles are capable of promoting changes in expression levels of genes involved in epithelial-mesenchymal transition and stem cell markers. Our findings suggest that secreted extracellular vesicles could represent potential diagnostic and/or prognostic markers for breast cancer and support a role for extracellular vesicles in cancer progression.
Conflict of interest statement
Figures
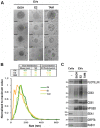
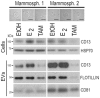
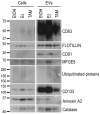
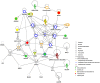


References
-
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, et al. (2000) Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology 141: 2982–2994. - PubMed
-
- Ali S, Coombes RC (2002) Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2: 101–112. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–111. - PubMed
-
- Vivanco M (2010) Function follows form: defining mammary stem cells. Sci Transl Med 2: 31ps22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

