Reduction of VLDL secretion decreases cholesterol excretion in niemann-pick C1-like 1 hepatic transgenic mice
- PMID: 24404162
- PMCID: PMC3880293
- DOI: 10.1371/journal.pone.0084418
Reduction of VLDL secretion decreases cholesterol excretion in niemann-pick C1-like 1 hepatic transgenic mice
Abstract
An effective way to reduce LDL cholesterol, the primary risk factor of atherosclerotic cardiovascular disease, is to increase cholesterol excretion from the body. Our group and others have recently found that cholesterol excretion can be facilitated by both hepatobiliary and transintestinal pathways. However, the lipoprotein that moves cholesterol through the plasma to the small intestine for transintestinal cholesterol efflux (TICE) is unknown. To test the hypothesis that hepatic very low-density lipoproteins (VLDL) support TICE, antisense oligonucleotides (ASO) were used to knockdown hepatic expression of microsomal triglyceride transfer protein (MTP), which is necessary for VLDL assembly. While maintained on a high cholesterol diet, Niemann-Pick C1-like 1 hepatic transgenic (L1Tg) mice, which predominantly excrete cholesterol via TICE, and wild type (WT) littermates were treated with control ASO or MTP ASO. In both WT and L1Tg mice, MTP ASO decreased VLDL triglyceride (TG) and cholesterol secretion. Regardless of treatment, L1Tg mice had reduced biliary cholesterol compared to WT mice. However, only L1Tg mice treated with MTP ASO had reduced fecal cholesterol excretion. Based upon these findings, we conclude that VLDL or a byproduct such as LDL can move cholesterol from the liver to the small intestine for TICE.
Conflict of interest statement
Figures
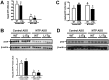
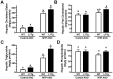
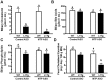
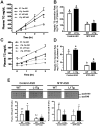

Similar articles
-
Hepatic NPC1L1 promotes hyperlipidemia in LDL receptor deficient mice.Biochem Biophys Res Commun. 2018 May 15;499(3):626-633. doi: 10.1016/j.bbrc.2018.03.200. Epub 2018 Mar 31. Biochem Biophys Res Commun. 2018. PMID: 29601818
-
The low density lipoprotein receptor prevents secretion of dense apoB100-containing lipoproteins from the liver.J Biol Chem. 2004 Jan 9;279(2):831-6. doi: 10.1074/jbc.M303057200. Epub 2003 Oct 28. J Biol Chem. 2004. PMID: 14583618
-
Differential impact of hepatic deficiency and total body inhibition of MTP on cholesterol metabolism and RCT in mice.J Lipid Res. 2014 May;55(5):816-25. doi: 10.1194/jlr.M042986. Epub 2014 Feb 7. J Lipid Res. 2014. PMID: 24511105 Free PMC article.
-
The role of factors that regulate the synthesis and secretion of very-low-density lipoprotein by hepatocytes.Crit Rev Clin Lab Sci. 1998 Dec;35(6):461-87. doi: 10.1080/10408369891234246. Crit Rev Clin Lab Sci. 1998. PMID: 9885772 Review.
-
Transintestinal cholesterol excretion in humans.Curr Opin Lipidol. 2018 Feb;29(1):10-17. doi: 10.1097/MOL.0000000000000473. Curr Opin Lipidol. 2018. PMID: 29189433 Review.
Cited by
-
XX sex chromosome complement promotes atherosclerosis in mice.Nat Commun. 2019 Jun 14;10(1):2631. doi: 10.1038/s41467-019-10462-z. Nat Commun. 2019. PMID: 31201301 Free PMC article.
-
Intestinal nuclear receptors in HDL cholesterol metabolism.J Lipid Res. 2015 Jul;56(7):1262-70. doi: 10.1194/jlr.R052704. Epub 2014 Jul 28. J Lipid Res. 2015. PMID: 25070952 Free PMC article. Review.
-
Simultaneous Determination of Biliary and Intestinal Cholesterol Secretion Reveals That CETP (Cholesteryl Ester Transfer Protein) Alters Elimination Route in Mice.Arterioscler Thromb Vasc Biol. 2019 Oct;39(10):1986-1995. doi: 10.1161/ATVBAHA.119.312952. Epub 2019 Aug 29. Arterioscler Thromb Vasc Biol. 2019. PMID: 31462090 Free PMC article.
-
A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion.Trends Pharmacol Sci. 2015 Jul;36(7):440-51. doi: 10.1016/j.tips.2015.04.002. Epub 2015 Apr 27. Trends Pharmacol Sci. 2015. PMID: 25930707 Free PMC article. Review.
-
Sustained and selective suppression of intestinal cholesterol synthesis by Ro 48-8071, an inhibitor of 2,3-oxidosqualene:lanosterol cyclase, in the BALB/c mouse.Biochem Pharmacol. 2014 Apr 1;88(3):351-63. doi: 10.1016/j.bcp.2014.01.031. Epub 2014 Jan 31. Biochem Pharmacol. 2014. PMID: 24486573 Free PMC article.
References
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. (2010) Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215. - PubMed
-
- Davis HR, Tershakovec AM, Tomassini JE, Musliner T (2011) Intestinal sterol transporters and cholesterol absorption inhibition. Curr Opin Lipidol 22: 467–478. - PubMed
-
- Brufau G, Groen AK, Kuipers F (2011) Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion. Arterioscler Thromb Vasc Biol 31: 1726–1733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

