T cells detect intracellular DNA but fail to induce type I IFN responses: implications for restriction of HIV replication
- PMID: 24404168
- PMCID: PMC3880311
- DOI: 10.1371/journal.pone.0084513
T cells detect intracellular DNA but fail to induce type I IFN responses: implications for restriction of HIV replication
Abstract
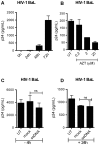
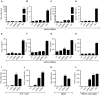
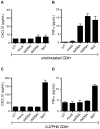
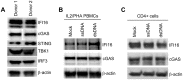
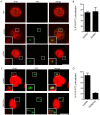
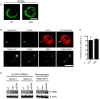
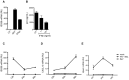
HIV infects key cell types of the immune system, most notably macrophages and CD4+ T cells. Whereas macrophages represent an important viral reservoir, activated CD4+ T cells are the most permissive cell types supporting high levels of viral replication. In recent years, it has been appreciated that the innate immune system plays an important role in controlling HIV replication, e.g. via interferon (IFN)-inducible restriction factors. Moreover, innate immune responses are involved in driving chronic immune activation and the pathogenesis of progressive immunodeficiency. Several pattern recognition receptors detecting HIV have been reported, including Toll-like receptor 7 and Retinoic-inducible gene-I, which detects viral RNA. Here we report that human primary T cells fail to induce strong IFN responses, despite the fact that this cell type does express key molecules involved in DNA signaling pathways. We demonstrate that the DNA sensor IFI16 migrates to sites of foreign DNA localization in the cytoplasm and recruits the signaling molecules stimulator of IFN genes and Tank-binding kinase, but this does not result in expression of IFN and IFN-stimulated genes. Importantly, we show that cytosolic DNA fails to affect HIV replication. However, exogenous treatment of activated T cells with type I IFN has the capacity to induce expression of IFN-stimulated genes and suppress HIV replication. Our data suggest the existence of an impaired DNA signaling machinery in T cells, which may prevent this cell type from activating cell-autonomous anti-HIV responses. This phenomenon could contribute to the high permissiveness of CD4+ T cells for HIV-1.
Conflict of interest statement
Figures
References
-
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, et al. (1983) Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220: 868–871. - PubMed
-
- De Cock KM, Jaffe HW, Curran JW (2012) The evolving epidemiology of HIV/AIDS. AIDS 26: 1205–1213. - PubMed
-
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, et al. (1995) Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373: 123–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

