Rab27a is essential for the formation of neutrophil extracellular traps (NETs) in neutrophil-like differentiated HL60 cells
- PMID: 24404184
- PMCID: PMC3880328
- DOI: 10.1371/journal.pone.0084704
Rab27a is essential for the formation of neutrophil extracellular traps (NETs) in neutrophil-like differentiated HL60 cells
Abstract
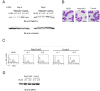
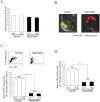
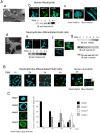
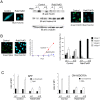
Neutrophils play a crucial role in host defence. In response to a variety of inflammatory stimulation, they form neutrophil extracellular traps (NETs). NETs are extracellular structures composed of chromatin fibers decorated with antimicrobial proteins and developing studies indicate that NETs contribute to extracellular microbial killing. While the intracellular signaling pathways that regulate NET formation remain largely unknown, there is growing evidence that generation of reactive oxygen species (ROS) is a key event for NET formation. The Rab family small GTPase Rab27a is an important component of the secretory machinery of azurophilic granules in neutrophils. However, the precise mechanism of NET formation and whether or not Rab27a contributes to this process are unknown. Using neutrophil-like differentiated HL60 cells, we show here that Rab27a plays an essential role in both phorbol myristate acetate (PMA)- and Candida albicans-induced NET formation by regulating ROS production. Rab27a-knockdown inhibited ROS-positive phagosome formation during complement-mediated phagocytosis. To investigate the role of Rab27a in neutrophil function in detail, both primary human neutrophils and neutrophil-like differentiated HL60 cells were treated with PMA, and NET formation process was assessed by measurement of release of histone H3 into the medium, citrullination of the arginine in position 3 of histone H4 and chase of the nuclear change of the living cells in the co-existence of both cell-permeable and -impermeable nuclear indicators. PMA-induced NET formation occured sequentially in both neutrophil-like differentiated HL60 cells and primary neutrophils, and Rab27a-knockdown clearly inhibited NET formation in association with reduced ROS production. We also found that serum-treated Candida albicans triggers NET formation in a ROS-dependent manner, and that Rab27a-knockdown inhibits this process as well. Our findings demonstrate that Rab27a plays an important role in NET formation induced by both Candida albicans infection and PMA treatment by regulating ROS production.
Conflict of interest statement
Figures
References
-
- Kuroda TS, Fukuda M (2004) Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol 6: 1195–1203. PubMed: 15543135. - PubMed
-
- Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, et al... (2000) Mutations in RAB27A Cause Griscelli Syndrome Associated with Haemophagocytic Syndrome. Nat Genet 25: 173–176. PubMed: 10835631. - PubMed
-
- Ménager MM, Ménasché G, Romao M, Knapnougel P, Ho CH, et al... (2007) Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13–4. Nat Immunol 8: 257–267. PubMed: 17237785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

