The interplay between regulatory T cells and metabolism in immune regulation
- PMID: 24404429
- PMCID: PMC3881602
- DOI: 10.4161/onci.26586
The interplay between regulatory T cells and metabolism in immune regulation
Abstract
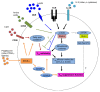
Regulatory T cells (Tregs) are crucial for peripheral tolerance and are intimately involved in immunological diseases and cancer. Recent studies have highlighted a key role for Tregs in metabolic disorders, for instance as they accumulate in the adipose tissue to protect against obesity-related inflammation and insulin resistance. Conversely, the generation and immunosuppressive functions of Tregs are influenced by both systemic and cellular metabolism. The nutritional status as well as metabolic cues such as those provided by leptin impinge upon the proliferation of Tregs. In addition, the mTOR-dependent lipid metabolism has a crucial role in programming the activity of Tregs under steady-state conditions as well as upon activation. This review discusses the intricate interaction between Tregs and metabolism, focusing on the roles of Tregs in systemic and local metabolic circuitries as well as on the regulation of Treg abundance and function by metabolic signals.
Keywords: FOXP3; Treg; lipid metabolism; mTOR; metabolism.
Figures
Similar articles
-
Metabolic control of regulatory T cell development and function.Trends Immunol. 2015 Jan;36(1):3-12. doi: 10.1016/j.it.2014.08.003. Epub 2014 Sep 20. Trends Immunol. 2015. PMID: 25248463 Free PMC article. Review.
-
Regulation of Treg Cell Metabolism and Function in Non-Lymphoid Tissues.Front Immunol. 2022 Jun 2;13:909705. doi: 10.3389/fimmu.2022.909705. eCollection 2022. Front Immunol. 2022. PMID: 35720275 Free PMC article. Review.
-
Short-term cold exposure supports human Treg induction in vivo.Mol Metab. 2019 Oct;28:73-82. doi: 10.1016/j.molmet.2019.08.002. Epub 2019 Aug 5. Mol Metab. 2019. PMID: 31427184 Free PMC article.
-
Interplay between mTOR and STAT5 signaling modulates the balance between regulatory and effective T cells.Immunobiology. 2015 Apr;220(4):510-7. doi: 10.1016/j.imbio.2014.10.020. Epub 2014 Oct 31. Immunobiology. 2015. PMID: 25468562
-
Metabolic Choice Tunes Foxp3+ Regulatory T Cell Function.Adv Exp Med Biol. 2021;1278:81-94. doi: 10.1007/978-981-15-6407-9_5. Adv Exp Med Biol. 2021. PMID: 33523444
Cited by
-
A Reflection of Metabolic Syndrome through the Window of COVID-19.Vaccines (Basel). 2022 Nov 19;10(11):1966. doi: 10.3390/vaccines10111966. Vaccines (Basel). 2022. PMID: 36423061 Free PMC article.
-
The Expression Quantitative Trait Loci in Immune Pathways and their Effect on Cutaneous Melanoma Prognosis.Clin Cancer Res. 2016 Jul 1;22(13):3268-80. doi: 10.1158/1078-0432.CCR-15-2066. Epub 2016 Jan 5. Clin Cancer Res. 2016. PMID: 26733611 Free PMC article.
-
Chronic ethanol consumption and HBV induce abnormal lipid metabolism through HBx/SWELL1/arachidonic acid signaling and activate Tregs in HBV-Tg mice.Theranostics. 2020 Jul 23;10(20):9249-9267. doi: 10.7150/thno.46005. eCollection 2020. Theranostics. 2020. PMID: 32802190 Free PMC article.
-
Epigenetic regulation of human FOXP3+ Tregs: from homeostasis maintenance to pathogen defense.Front Immunol. 2024 Jul 31;15:1444533. doi: 10.3389/fimmu.2024.1444533. eCollection 2024. Front Immunol. 2024. PMID: 39144146 Free PMC article. Review.
-
Leptin Deficiency May Influence the Divergence of Cell-Mediated Immunity Between Lepromatous and Tuberculoid Leprosy Patients.J Inflamm Res. 2022 Dec 13;15:6719-6728. doi: 10.2147/JIR.S389845. eCollection 2022. J Inflamm Res. 2022. PMID: 36536644 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
