Aldehyde dehydrogenase and ATP binding cassette transporter G2 (ABCG2) functional assays isolate different populations of prostate stem cells where ABCG2 function selects for cells with increased stem cell activity
- PMID: 24405526
- PMCID: PMC3854760
- DOI: 10.1186/scrt343
Aldehyde dehydrogenase and ATP binding cassette transporter G2 (ABCG2) functional assays isolate different populations of prostate stem cells where ABCG2 function selects for cells with increased stem cell activity
Abstract
Introduction: High expression of aldehyde dehydrogenase1A1 (ALDH1A1) is observed in many organs and tumors and may identify benign and cancer stem cell populations.
Methods: In the current study, the stem cell characteristics were determined in cells isolated from human prostate cell lines and clinical prostate specimens based upon the ALDEFLUOR™ assay. Cells isolated based on the ALDEFLUOR™ assay were compared to cells isolated based on ATP binding cassette transporter G2 (ABCG2) activity using the side population assay. To test for stem cell characteristics of self-renewal and multipotency, cells with high and low ALDH1A1 activity, based on the ALDEFLUOR™ assay (ALDHHi and ALDH Low), were isolated from prostate clinical specimens and were recombined with rat urogenital sinus mesenchyme to induce prostate gland formation.
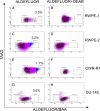
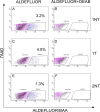
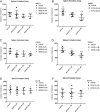
Results: The percentage of ALDH Hi cells in prostate cell lines (RWPE-1, RWPE-2, CWR-R1, and DU-145) was 0.5 to 6%, similarly in non-tumor and tumor clinical specimens the percentage of ALDH Hi cells was 0.6 to 4%. Recombinants using ALDH Hi cells serially generated prostate tissue up to three generations with as few as 250 starting cells. Immunohistochemical analysis of the recombinants using ALDHHi cells contained prostatic glands frequently expressing androgen receptor (AR), p63, chromogranin A, ALDH1A1, ABCG2, and prostate specific antigen (PSA), compared to their ALDH Low counterparts. Inhibition of ALDH resulted in the reduction of sphere formation capabilities in the CWR-R1, but not in the RWPE-2 and DU-145, prostate cell lines. ABCG2 inhibition resulted in a more robust decrease of sphere formation in androgen sensitive cell lines, CWR-R1 and RWPE-2, but not androgen insensitive DU-145. ALDH1A1 expression was enriched in ALDH Hi cells and non-side population cells. ABCG2 expression was only enriched in side population cells.
Conclusions: The percentage of ALDHHi cells in prostate cell lines and prostate tissue was consistently higher compared to cells with high ABCG2 activity, identified with the side population assay. The expression of the stem and differentiation markers indicates the ALDH Hi recombinants contained cells with self-renewal and multipotency activity. When the two assays were directly compared, cells with the side population phenotype demonstrated more stem cell potential in the tissue recombination assay compared to ALDH Hi cells. The increased stem cell potential of side population cells in the tissue recombination assay and the decrease in sphere formation when ABCG2 is inhibited indicates that the side population enriches for prostate stem cells.
Figures
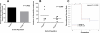



Similar articles
-
Human prostate side population cells demonstrate stem cell properties in recombination with urogenital sinus mesenchyme.PLoS One. 2013;8(1):e55062. doi: 10.1371/journal.pone.0055062. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383057 Free PMC article.
-
ABCG2-mediated DyeCycle Violet efflux defined side population in benign and malignant prostate.Cell Cycle. 2009 Apr 1;8(7):1053-61. doi: 10.4161/cc.8.7.8043. Epub 2009 Apr 30. Cell Cycle. 2009. PMID: 19270533 Free PMC article.
-
Esophageal cancer tumorspheres involve cancer stem-like populations with elevated aldehyde dehydrogenase enzymatic activity.Mol Med Rep. 2012 Sep;6(3):519-24. doi: 10.3892/mmr.2012.939. Epub 2012 Jun 8. Mol Med Rep. 2012. PMID: 22684859
-
ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy.Life Sci. 2010 Apr 24;86(17-18):631-7. doi: 10.1016/j.lfs.2010.02.012. Epub 2010 Feb 14. Life Sci. 2010. PMID: 20159023 Review.
-
Multidrug resistance in cancer chemotherapy and xenobiotic protection mediated by the half ATP-binding cassette transporter ABCG2.Curr Med Chem Anticancer Agents. 2004 Jan;4(1):31-42. doi: 10.2174/1568011043482205. Curr Med Chem Anticancer Agents. 2004. PMID: 14754410 Review.
Cited by
-
High Skp2 expression is associated with a mesenchymal phenotype and increased tumorigenic potential of prostate cancer cells.Sci Rep. 2019 Apr 5;9(1):5695. doi: 10.1038/s41598-019-42131-y. Sci Rep. 2019. PMID: 30952903 Free PMC article.
-
Uric acid: a modulator of prostate cells and activin sensitivity.Mol Cell Biochem. 2016 Mar;414(1-2):187-99. doi: 10.1007/s11010-016-2671-8. Epub 2016 Feb 24. Mol Cell Biochem. 2016. PMID: 26910779
-
Identification of key genes and specific pathways potentially involved in androgen-independent, mitoxantrone-resistant prostate cancer.Cancer Manag Res. 2019 Jan 3;11:419-430. doi: 10.2147/CMAR.S179467. eCollection 2019. Cancer Manag Res. 2019. PMID: 30655694 Free PMC article.
-
Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer.Int J Mol Sci. 2016 Jul 19;17(7):1163. doi: 10.3390/ijms17071163. Int J Mol Sci. 2016. PMID: 27447616 Free PMC article. Review.
-
Metformin and prostate cancer stem cells: a novel therapeutic target.Prostate Cancer Prostatic Dis. 2015 Dec;18(4):303-9. doi: 10.1038/pcan.2015.35. Epub 2015 Jul 28. Prostate Cancer Prostatic Dis. 2015. PMID: 26215782 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

