Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration
- PMID: 24405740
- PMCID: PMC4109727
- DOI: 10.1016/j.ophtha.2013.10.037
Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration
Abstract
Purpose: We sought to determine the most cost-effective treatment for patients with newly diagnosed neovascular macular degeneration: monthly or as-needed bevacizumab injections, or monthly or as-needed ranibizumab injections.
Design: Cost-effectiveness analysis.
Participants: Hypothetical cohort of 80-year-old patients with newly diagnosed neovascular macular degeneration.
Methods: Using a mathematical model with a 20-year time horizon, we compared the incremental cost-effectiveness of treating a hypothetical cohort of 80-year-old patients with newly diagnosed neovascular macular degeneration using monthly bevacizumab, as-needed bevacizumab, monthly ranibizumab, or as-needed ranibizumab. Data came from the Comparison of Age-related macular degeneration Treatment Trial (CATT), the Medicare Fee Schedule, and the medical literature.
Main outcome measures: Costs, quality-adjusted life-years (QALYs), and incremental costs per QALY gained.
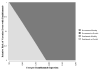
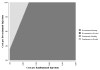
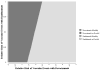
Results: Compared with as-needed bevacizumab, the incremental cost-effectiveness ratio of monthly bevacizumab is $24,2 357/QALY. Monthly ranibizumab gains an additional 0.02 QALYs versus monthly bevacizumab at an incremental cost-effectiveness ratio of >$10 million/QALY. As-needed ranibizumab was dominated by monthly bevacizumab, meaning it was more costly and less effective. In sensitivity analyses assuming a willingness to pay of $100,000/QALY, the annual risk of serious vascular events would have to be ≥2.5 times higher with bevacizumab than that observed in the CATT trial for as-needed ranibizumab to have an incremental cost-effectiveness ratio of <$100,000/QALY. In another sensitivity analysis, even if every patient receiving bevacizumab experienced declining vision by 1 category (e.g., from 20/25-20/40 to 20/50-20/80) after 2 years but every patient receiving ranibizumab retained their vision level, as-needed ranibizumab would have an incremental cost-effectiveness ratio of $97,340/QALY.
Conclusions: Even after considering the potential for differences in risks of serious adverse events and therapeutic effectiveness, bevacizumab confers considerably greater value than ranibizumab for the treatment of neovascular macular degeneration.
Copyright © 2014 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- National Advisory Eye Council. Vision Research, a National Plan, 1994–1998. Washington, D.C: U.S. Department of Health and Human Services; 1993. pp. xx–xx. NIH publication 93–3186.
-
- Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. - PubMed
-
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. - PubMed
-
- Scilley K, Jackson GR, Cideciyan AV, et al. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109:1235–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

