Tracking of stem cells in vivo for cardiovascular applications
- PMID: 24406054
- PMCID: PMC3925252
- DOI: 10.1186/1532-429X-16-7
Tracking of stem cells in vivo for cardiovascular applications
Abstract
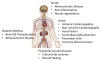
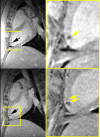
In the past ten years, the concept of injecting stem and progenitor cells to assist with rebuilding damaged blood vessels and myocardial tissue after injury in the heart and peripheral vasculature has moved from bench to bedside. Non-invasive imaging can not only provide a means to assess cardiac repair and, thereby, cellular therapy efficacy but also a means to confirm cell delivery and engraftment after administration. In this first of a two-part review, we will review the different types of cellular labeling techniques and the application of these techniques in cardiovascular magnetic resonance and ultrasound. In addition, we provide a synopsis of the cardiac cellular clinical trials that have been performed to-date.
Figures
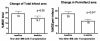
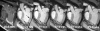


Similar articles
-
In vivo tracking in cardiac stem cell-based therapy.Prog Cardiovasc Dis. 2007 May-Jun;49(6):414-20. doi: 10.1016/j.pcad.2007.02.005. Prog Cardiovasc Dis. 2007. PMID: 17498521 Review.
-
Stem cell labeling for noninvasive delivery and tracking in cardiovascular regenerative therapy.Expert Rev Cardiovasc Ther. 2010 Aug;8(8):1149-60. doi: 10.1586/erc.10.106. Expert Rev Cardiovasc Ther. 2010. PMID: 20670192 Free PMC article. Review.
-
Stem cell therapy in cardiovascular disorders.Cardiovasc Ther. 2010 Oct;28(5):e101-10. doi: 10.1111/j.1755-5922.2010.00208.x. Cardiovasc Ther. 2010. PMID: 21050418 Review.
-
Magnetic Resonance Imaging of Iron Oxide-Labeled Human Embryonic Stem Cell-Derived Cardiac Progenitors.Stem Cells Transl Med. 2016 Jan;5(1):67-74. doi: 10.5966/sctm.2015-0077. Epub 2015 Nov 18. Stem Cells Transl Med. 2016. PMID: 26582908 Free PMC article.
-
Advances in cardiovascular molecular imaging for tracking stem cell therapy.Thromb Haemost. 2010 Jul;104(1):13-22. doi: 10.1160/TH09-08-0530. Epub 2010 May 10. Thromb Haemost. 2010. PMID: 20458434 Free PMC article. Review.
Cited by
-
Macroscopic fluorescence imaging: a novel technique to monitor retention and distribution of injected microspheres in an experimental model of ischemic heart failure.PLoS One. 2014 Aug 4;9(8):e101775. doi: 10.1371/journal.pone.0101775. eCollection 2014. PLoS One. 2014. PMID: 25089764 Free PMC article.
-
Review of Journal of Cardiovascular Magnetic Resonance 2014.J Cardiovasc Magn Reson. 2015 Nov 20;17:99. doi: 10.1186/s12968-015-0203-4. J Cardiovasc Magn Reson. 2015. PMID: 26589839 Free PMC article. Review.
-
Cardiac shock wave therapy promotes arteriogenesis of coronary micrangium, and ILK is involved in the biomechanical effects by proteomic analysis.Sci Rep. 2018 Jan 29;8(1):1814. doi: 10.1038/s41598-018-19393-z. Sci Rep. 2018. PMID: 29379038 Free PMC article.
-
Magnetically Responsive Bone Marrow Mesenchymal Stem Cell-Derived Smooth Muscle Cells Maintain Their Benefits to Augmenting Elastic Matrix Neoassembly.Tissue Eng Part C Methods. 2016 Apr;22(4):301-11. doi: 10.1089/ten.TEC.2015.0349. Epub 2016 Mar 18. Tissue Eng Part C Methods. 2016. PMID: 26830683 Free PMC article.
-
Epicardium-Derived Cells Formed After Myocardial Injury Display Phagocytic Activity Permitting In Vivo Labeling and Tracking.Stem Cells Transl Med. 2016 May;5(5):639-50. doi: 10.5966/sctm.2015-0159. Epub 2016 Apr 7. Stem Cells Transl Med. 2016. PMID: 27057005 Free PMC article.
References
-
- Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schonberg SO, Debl K, Strohm O, Ahlstrom H. et al.Superior diagnostic performance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: The secondary endpoints of the multicenter multivendor MR-IMPACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial) J Cardiovasc Magn Reson. 2012;14:61. - PMC - PubMed
-
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW. et al.Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. - PubMed
-
- Leistner DM, Fischer-Rasokat U, Honold J, Seeger FH, Schachinger V, Lehmann R, Martin H, Burck I, Urbich C, Dimmeler S. et al.Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011;100:925–934. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

