Connexin36 identified at morphologically mixed chemical/electrical synapses on trigeminal motoneurons and at primary afferent terminals on spinal cord neurons in adult mouse and rat
- PMID: 24406437
- PMCID: PMC3951135
- DOI: 10.1016/j.neuroscience.2013.12.057
Connexin36 identified at morphologically mixed chemical/electrical synapses on trigeminal motoneurons and at primary afferent terminals on spinal cord neurons in adult mouse and rat
Abstract
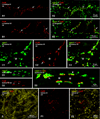
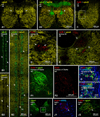
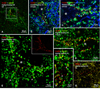
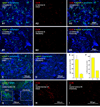
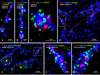
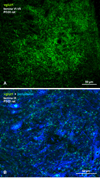
Morphologically mixed chemical/electrical synapses at axon terminals, with the electrical component formed by gap junctions, is common in the CNS of lower vertebrates. In mammalian CNS, evidence for morphologically mixed synapses has been obtained in only a few locations. Here, we used immunofluorescence approaches to examine the localization of the neuronally expressed gap junction forming protein connexin36 (Cx36) in relation to the axon terminal marker vesicular glutamate transporter-1 (vglut1) in the spinal cord and the trigeminal motor nucleus (Mo5) of rat and mouse. In adult rodents, immunolabeling for Cx36 appeared exclusively as Cx36-puncta, and was widely distributed at all rostro-caudal levels in most spinal cord laminae and in the Mo5. A high proportion of Cx36-puncta was co-localized with vglut1, forming morphologically mixed synapses on motoneurons, in intermediate spinal cord lamina, and in regions of medial lamina VII, where vglut1-containing terminals associated with Cx36 converged on neurons adjacent to the central canal. Unilateral transection of lumbar dorsal roots reduced immunolabeling of both vglut1 and Cx36 in intermediate laminae and lamina IX. Further, vglut1-terminals displaying Cx36-puncta were contacted by terminals labeled for glutamic acid decarboxylase65, which is known to be contained in presynaptic terminals on large-diameter primary afferents. Developmentally, mixed synapses begin to emerge in the spinal cord only after the second to third postnatal week and thereafter increase to adult levels. Our findings demonstrate that axon terminals of primary afferent origin form morphologically mixed synapses containing Cx36 in broadly distributed areas of adult rodent spinal cord and Mo5.
Keywords: gap junctions; interneurons; motoneurons; vesicular glutamate transporter-1.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures






References
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. - PubMed
-
- Arasaki K, Kudo N, Nakanishi T. Firing of spinal motoneurons due to electrical interactions in the rat: an in vitro study. Exp Brain Res. 1984;54:437–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

