Coenzyme Q supplementation or over-expression of the yeast Coq8 putative kinase stabilizes multi-subunit Coq polypeptide complexes in yeast coq null mutants
- PMID: 24406904
- PMCID: PMC3959571
- DOI: 10.1016/j.bbalip.2013.12.017
Coenzyme Q supplementation or over-expression of the yeast Coq8 putative kinase stabilizes multi-subunit Coq polypeptide complexes in yeast coq null mutants
Abstract
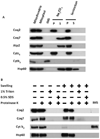
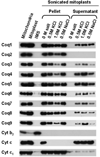
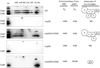
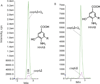
Coenzyme Q biosynthesis in yeast requires a multi-subunit Coq polypeptide complex. Deletion of any one of the COQ genes leads to respiratory deficiency and decreased levels of the Coq4, Coq6, Coq7, and Coq9 polypeptides, suggesting that their association in a high molecular mass complex is required for stability. Over-expression of the putative Coq8 kinase in certain coq null mutants restores steady-state levels of the sensitive Coq polypeptides and promotes the synthesis of late-stage Q-intermediates. Here we show that over-expression of Coq8 in yeast coq null mutants profoundly affects the association of several of the Coq polypeptides in high molecular mass complexes, as assayed by separation of digitonin extracts of mitochondria by two-dimensional blue-native/SDS PAGE. The Coq4 polypeptide persists at high molecular mass with over-expression of Coq8 in coq3, coq5, coq6, coq7, coq9, and coq10 mutants, indicating that Coq4 is a central organizer of the Coq complex. Supplementation with exogenous Q6 increased the steady-state levels of Coq4, Coq7, and Coq9, and several other mitochondrial polypeptides in select coq null mutants, and also promoted the formation of late-stage Q-intermediates. Q supplementation may stabilize this complex by interacting with one or more of the Coq polypeptides. The stabilizing effects of exogenously added Q6 or over-expression of Coq8 depend on Coq1 and Coq2 production of a polyisoprenyl intermediate. Based on the observed interdependence of the Coq polypeptides, the effect of exogenous Q6, and the requirement for an endogenously produced polyisoprenyl intermediate, we propose a new model for the Q-biosynthetic complex, termed the CoQ-synthome.
Keywords: Coenzyme Q supplementation; Mitochondrial metabolism; Protein complex; Q-biosynthetic intermediate; Saccharomyces cerevisiae; Ubiquinone.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures







References
-
- Crane FL, Barr R. Chemical structure and properties of coenzyme Q and related compounds. In: Lenaz G, editor. Coenzyme Q: Biochemistry, bioenergetics and clinical applications of ubiquinone. New York, NY: John Wiley & Sons; 1985. pp. 1–37.
-
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta. 2004;1660:171–199. - PubMed
-
- Lenaz G, De Santis A. A survey of the function and specificity of ubiquinone in the mitochondrial respiratory chain. In: Lenaz G, editor. Coenzyme Q Biochemistry, Bioenergetics and Clinical Applications of Ubiquinone. New York: John Wiley & Sons; 1985. pp. 165–199.
-
- Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. The FEBS journal. 2008;275:3352–3361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

