Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice
- PMID: 24407029
- PMCID: PMC3966977
- DOI: 10.1161/ATVBAHA.113.303044
Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice
Abstract
Objective: Preclinical and clinical studies have shown beneficial effects of infusions of apolipoprotein A-I (ApoA-I) on atherosclerosis. ApoA-I is also a target for myeloperoxidase-mediated oxidation, leading in vitro to a loss of its ability to promote ATP-binding cassette transporter A1-dependent macrophage cholesterol efflux. Therefore, we hypothesized that myeloperoxidase-mediated ApoA-I oxidation would impair its promotion of reverse cholesterol transport in vivo and the beneficial effects on atherosclerotic plaques.
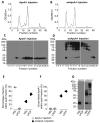
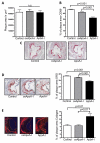
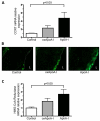
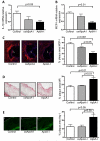
Approach and results: ApoA-I(-/-) or apolipoprotein E-deficient mice were subcutaneously injected with native human ApoA-I, oxidized human ApoA-I (myeloperoxidase/hydrogen peroxide/chloride treated), or carrier. Although early postinjection (8 hours) levels of total ApoA-I in plasma were similar for native versus oxidized human ApoA-I, native ApoA-I primarily resided within the high-density lipoprotein fraction, whereas the majority of oxidized human ApoA-I was highly cross-linked and not high-density lipoprotein particle associated, consistent with impaired ATP-binding cassette transporter A1 interaction. In ApoA-I(-/-) mice, ApoA-I oxidation significantly impaired reverse cholesterol transport in vivo. In advanced aortic root atherosclerotic plaques of apolipoprotein E-deficient mice, native ApoA-I injections led to significant decreases in lipid content, macrophage number, and an increase in collagen content; in contrast, oxidized human ApoA-I failed to mediate these changes. The decrease in plaque macrophages with native ApoA-I was accompanied by significant induction of their chemokine receptor CCR7. Furthermore, only native ApoA-I injections led to a significant reduction of inflammatory M1 and increase in anti-inflammatory M2 macrophage markers in the plaques.
Conclusions: Myeloperoxidase-mediated oxidation renders ApoA-I dysfunctional and unable to (1) promote reverse cholesterol transport, (2) mediate beneficial changes in the composition of atherosclerotic plaques, and (3) pacify the inflammatory status of plaque macrophages.
Keywords: apolipoprotein A-I; atherosclerosis; myeloperoxidase.
Figures

Comment in
-
Myeloperoxidase-mediated dysfunctional high-density lipoprotein.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):695-6. doi: 10.1161/ATVBAHA.114.303282. Arterioscler Thromb Vasc Biol. 2014. PMID: 24665120 No abstract available.
References
-
- Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L, Chaabane L, Morisetti A, Lorusso V, Martin BJ, Bisgaier CL, Krause B, Newton RS, Sirtori CR, Chiesa G. Dose-related effects of repeated etc-216 (recombinant apolipoprotein a-i milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: In vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51:1098–1103. - PubMed
-
- Ibanez B, Vilahur G, Cimmino G, Speidl WS, Pinero A, Choi BG, Zafar MU, Santos-Gallego CG, Krause B, Badimon L, Fuster V, Badimon JJ. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein a-i milano (etc-216) administration: Magnetic resonance imaging study in an experimental model of atherosclerosis. J Am Coll Cardiol. 2008;51:1104–1109. - PubMed
-
- Giannarelli C, Cimmino G, Ibanez B, Chiesa G, Garcia-Prieto J, Santos-Gallego CG, Alique-Aguilar M, Fuster V, Sirtori C, Badimon JJ. Acute apoa-i milano administration induces plaque regression and stabilisation in the long term. Thrombosis and haemostasis. 2012;108:1246–1248. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

