Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD)
- PMID: 24407427
- PMCID: PMC3971993
- DOI: 10.1007/s00401-013-1238-y
Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD)
Erratum in
-
Erratum to: Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD).Acta Neuropathol. 2015 Jun;129(6):929. doi: 10.1007/s00401-015-1428-x. Acta Neuropathol. 2015. PMID: 25931052 No abstract available.
Abstract
We examined regional distribution patterns of phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) intraneuronal inclusions in frontotemporal lobar degeneration (FTLD). Immunohistochemistry was performed on 70 μm sections from FTLD-TDP autopsy cases (n = 39) presenting with behavioral variant frontotemporal dementia. Two main types of cortical pTDP-43 pathology emerged, characterized by either predominantly perikaryal pTDP-43 inclusions (cytoplasmic type, cFTLD) or long aggregates in dendrites (neuritic type, nFTLD). Cortical involvement in nFTLD was extensive and frequently reached occipital areas, whereas cases with cFTLD often involved bulbar somatomotor neurons and the spinal cord. We observed four patterns indicative of potentially sequential dissemination of pTDP-43: cases with the lowest burden of pathology (pattern I) were characterized by widespread pTDP-43 lesions in the orbital gyri, gyrus rectus, and amygdala. With increasing burden of pathology (pattern II) pTDP-43 lesions emerged in the middle frontal and anterior cingulate gyrus as well as in anteromedial temporal lobe areas, the superior and medial temporal gyri, striatum, red nucleus, thalamus, and precerebellar nuclei. More advanced cases showed a third pattern (III) with involvement of the motor cortex, bulbar somatomotor neurons, and the spinal cord anterior horn, whereas cases with the highest burden of pathology (pattern IV) were characterized by pTDP-43 lesions in the visual cortex. We interpret the four neuropathological patterns in bvFTD to be consistent with the hypothesis that pTDP-43 pathology can spread sequentially and may propagate along axonal pathways.
Figures
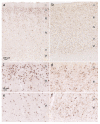

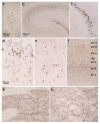
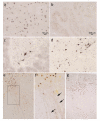
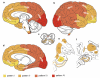
References
-
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Ann N Y Acad Sci. 2007;1121:10–32. - PubMed
-
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. - PubMed
-
- Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991;313:65–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG010124/AG/NIA NIH HHS/United States
- K08 AG039510/AG/NIA NIH HHS/United States
- AG010124/AG/NIA NIH HHS/United States
- K08 AG033101/AG/NIA NIH HHS/United States
- AG039510/AG/NIA NIH HHS/United States
- NS044266/NS/NINDS NIH HHS/United States
- K23 NS088341/NS/NINDS NIH HHS/United States
- R01 NS044266/NS/NINDS NIH HHS/United States
- P01 AG032953/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- AG017586/AG/NIA NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- T32-AG000255/AG/NIA NIH HHS/United States
- AG032953/AG/NIA NIH HHS/United States
- AG033101/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

