Metabolic biology of 3-methylglutaconic acid-uria: a new perspective
- PMID: 24407466
- PMCID: PMC4016128
- DOI: 10.1007/s10545-013-9669-0
Metabolic biology of 3-methylglutaconic acid-uria: a new perspective
Abstract
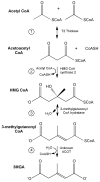
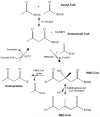
Over the past 25 years a growing number of distinct syndromes/mutations associated with compromised mitochondrial function have been identified that share a common feature: urinary excretion of 3-methylglutaconic acid (3MGA). In the leucine degradation pathway, carboxylation of 3-methylcrotonyl CoA leads to formation of 3-methylglutaconyl CoA while 3-methylglutaconyl CoA hydratase converts this metabolite to 3-hydroxy-3-methylglutaryl CoA (HMG CoA). In "primary" 3MGA-uria, mutations in the hydratase are directly responsible for the accumulation of 3MGA. On the other hand, in all "secondary" 3MGA-urias, no defect in leucine catabolism exists and the metabolic origin of 3MGA is unknown. Herein, a path to 3MGA from mitochondrial acetyl CoA is proposed. The pathway is initiated when syndrome-associated mutations/DNA deletions result in decreased Krebs cycle flux. When this occurs, acetoacetyl CoA thiolase condenses two acetyl CoA into acetoacetyl CoA plus CoASH. Subsequently, HMG CoA synthase 2 converts acetoacetyl CoA and acetyl CoA to HMG CoA. Under syndrome-specific metabolic conditions, 3-methylglutaconyl CoA hydratase converts HMG CoA into 3-methylglutaconyl CoA in a reverse reaction of the leucine degradation pathway. This metabolite fails to proceed further up the leucine degradation pathway owing to the kinetic properties of 3-methylcrotonyl CoA carboxylase. Instead, hydrolysis of the CoA moiety of 3-methylglutaconyl CoA generates 3MGA, which appears in urine. If experimentally confirmed, this pathway provides an explanation for the occurrence of 3MGA in multiple disorders associated with compromised mitochondrial function.
Figures

Similar articles
-
On the origin of 3-methylglutaconic acid in disorders of mitochondrial energy metabolism.J Inherit Metab Dis. 2016 Sep;39(5):749-756. doi: 10.1007/s10545-016-9933-1. Epub 2016 Apr 18. J Inherit Metab Dis. 2016. PMID: 27091556 Free PMC article.
-
Inborn errors of metabolism with 3-methylglutaconic aciduria as discriminative feature: proper classification and nomenclature.J Inherit Metab Dis. 2013 Nov;36(6):923-8. doi: 10.1007/s10545-012-9580-0. Epub 2013 Jan 8. J Inherit Metab Dis. 2013. PMID: 23296368 Review.
-
Inborn errors of metabolism associated with 3-methylglutaconic aciduria.Clin Chim Acta. 2021 Nov;522:96-104. doi: 10.1016/j.cca.2021.08.016. Epub 2021 Aug 16. Clin Chim Acta. 2021. PMID: 34411555 Free PMC article. Review.
-
Fungal metabolic model for type I 3-methylglutaconic aciduria.J Biol Chem. 2004 Jul 30;279(31):32385-92. doi: 10.1074/jbc.M313044200. Epub 2004 Jun 4. J Biol Chem. 2004. PMID: 15181004
-
Deficiency of 3-methylglutaconyl-coenzyme A hydratase in two siblings with 3-methylglutaconic aciduria.J Clin Invest. 1986 Apr;77(4):1148-52. doi: 10.1172/JCI112415. J Clin Invest. 1986. PMID: 3082934 Free PMC article.
Cited by
-
Barth Syndrome: Connecting Cardiolipin to Cardiomyopathy.Lipids. 2017 Feb;52(2):99-108. doi: 10.1007/s11745-016-4229-7. Epub 2017 Jan 9. Lipids. 2017. PMID: 28070695 Free PMC article. Review.
-
Metabolic annotation of 2-ethylhydracrylic acid.Clin Chim Acta. 2015 Aug 25;448:91-7. doi: 10.1016/j.cca.2015.06.012. Epub 2015 Jun 23. Clin Chim Acta. 2015. PMID: 26115894 Free PMC article. Review.
-
International Workshop:: Outcome measures and clinical trial readiness in primary mitochondrial myopathies in children and adults. Consensus recommendations. 16-18 November 2016, Rome, Italy.Neuromuscul Disord. 2017 Dec;27(12):1126-1137. doi: 10.1016/j.nmd.2017.08.006. Epub 2017 Sep 8. Neuromuscul Disord. 2017. PMID: 29074296 Free PMC article. No abstract available.
-
Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society.Genet Med. 2015 Sep;17(9):689-701. doi: 10.1038/gim.2014.177. Epub 2014 Dec 11. Genet Med. 2015. PMID: 25503498 Free PMC article. Review.
-
The Effects of Hospitalisation on the Serum Metabolome in COVID-19 Patients.Metabolites. 2023 Aug 16;13(8):951. doi: 10.3390/metabo13080951. Metabolites. 2023. PMID: 37623894 Free PMC article.
References
-
- Bissler JJ, Tsoras M, Goring HH, et al. Infantile dilated X-linked cardiomyopathy, G4.5 mutations, altered lipids, and ultrastructural malformations of mitochondria in heart, liver, and skeletal muscle. Lab Invest. 2002;82(3):335–344. - PubMed
-
- Bird MJ, Thorburn DR, Frazier AE. Modelling biochemical features of mitochondrial neuropathology. Biochim Biophys Acta. 2013 Oct 22; Epub ahead of print. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

