Factors associated with early progression of non-small-cell lung cancer treated by epidermal growth factor receptor tyrosine-kinase inhibitors
- PMID: 24408092
- PMCID: PMC3930390
- DOI: 10.1002/cam4.180
Factors associated with early progression of non-small-cell lung cancer treated by epidermal growth factor receptor tyrosine-kinase inhibitors
Abstract
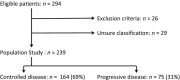
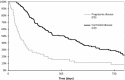
Epidermal growth factor receptor tyrosine-kinase inhibitors (EGFR-TKI) are a therapeutic option as second-line therapy in non-small-cell lung carcinoma (NSCLC), regardless of the EGFR gene status. Identifying patients with early progression during EGFR-TKI treatment will help clinicians to choose the best regimen, TKI or chemotherapy. From a prospective database, all patients treated with gefitinib or erlotinib between 2001 and 2010 were retrospectively reviewed. Patients were classified into two groups according to their tumor response by RECIST after 45 days of treatment, progressive disease (PD) or controlled disease (CD). Two hundred and sixty-eight patients were treated with EGFR-TKI, among whom 239 were classified as PD (n = 75) and CD (n = 164). Median overall survival was 77 days (95% CI 61-109) for PD and 385 days (95% CI 267-481) for CD. Patients with PD were of younger age (P = 0.004) and more frequently current smokers (P = 0.001) had more frequently a performance status ≥2 (P = 0.012), a weight loss ≥10% (P = 0.025), a shorter time since diagnosis (P < 0.0001), a pathological classification as non-otherwise-specified NSCLC (P = 0.01), and the presence of abdominal metastases (P = 0.008). In multivariate analysis, abdominal metastases were the only factor associated with early progression (odds ratio (OR) 2.17, 95% CI [1.12-4.19]; P = 0.021). Wild-type EGFR versus mutated EGFR was associated with early progression. The presence of abdominal metastasis was independently associated with early progression in metastatic NSCLC receiving EGFR-TKI.
Keywords: Epidermal growth factor receptor tyrosine-kinase inhibitors; erlotinib; gefitinib; non-small-cell lung cancer; progressive disease.
© 2014 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. - PubMed
-
- Muruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J. Clin. Oncol. 2008;26:4244–4252. - PubMed
-
- Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small-cell lung cancer patients who have previously received platinum-based chemotherapy. Clin. Cancer Res. 2010;16:1307–1314. - PubMed
-
- Vamvakas L, Agelaki S, Kentepozidis K, Karampeazis A, Pallis AG, Christophyllakis C, et al. Pemetrexed (MTA) compared with erlotinib (ERL) in pretreated patients with advanced non-small cell lung cancer (NSCLC): results of a randomized phase III Hellenic Oncology Research Group Trial. J. Clin. Oncol. 2010;28(Suppl. 15s):abstr 7519.
-
- Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

