Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD
- PMID: 24408120
- PMCID: PMC3944759
- DOI: 10.2215/CJN.05170513
Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD
Abstract
Background and objectives: Ferric citrate hydrate is a novel iron-based phosphate binder being developed for hyperphosphatemia in patients with CKD.
Design, setting, participants, & measurements: A phase 3, multicenter, randomized, double blind, placebo-controlled study investigated the efficacy and safety of ferric citrate hydrate in nondialysis-dependent patients with CKD. Starting in April of 2011, 90 CKD patients (eGFR=9.21±5.72 ml/min per 1.73 m(2)) with a serum phosphate≥5.0 mg/dl were randomized 2:1 to ferric citrate hydrate or placebo for 12 weeks. The primary end point was change in serum phosphate from baseline to the end of treatment. Secondary end points included the percentage of patients achieving target serum phosphate levels (2.5-4.5 mg/dl) and change in fibroblast growth factor-23 at the end of treatment.
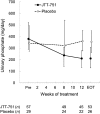
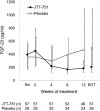
Results: The mean change in serum phosphate was -1.29 mg/dl (95% confidence interval, -1.63 to -0.96 mg/dl) in the ferric citrate hydrate group and 0.06 mg/dl (95% confidence interval, -0.20 to 0.31 mg/dl) in the placebo group (P<0.001 for difference between groups). The percentage of patients achieving target serum phosphate levels was 64.9% in the ferric citrate hydrate group and 6.9% in the placebo group (P<0.001). Fibroblast growth factor-23 concentrations were significantly lower in patients treated with ferric citrate hydrate versus placebo (change from baseline [median], -142.0 versus 67.0 pg/ml; P<0.001). Ferric citrate hydrate significantly increased serum iron, ferritin, and transferrin saturation compared with placebo (P=0.001 or P<0.001). Five patients discontinued active treatment because of treatment-emergent adverse events with ferric citrate hydrate treatment versus one patient with placebo. Overall, adverse drug reactions were similar in patients receiving ferric citrate hydrate or placebo, with gastrointestinal disorders occurring in 30.0% of ferric citrate hydrate patients and 26.7% of patients receiving placebo.
Conclusion: In patients with nondialysis-dependent CKD, 12-week treatment with ferric citrate hydrate resulted in significant reductions in serum phosphate and fibroblast growth factor-23 while simultaneously increasing serum iron parameters.
Figures

References
-
- Ritz E, Gross ML: Hyperphosphatemia in renal failure. Blood Purif 23: 6–9, 2005 - PubMed
-
- Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 - PubMed
-
- Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 - PMC - PubMed
-
- Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 - PubMed
-
- Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

