Lithium causes G2 arrest of renal principal cells
- PMID: 24408872
- PMCID: PMC3935595
- DOI: 10.1681/ASN.2013090988
Lithium causes G2 arrest of renal principal cells
Abstract
Vasopressin-regulated expression and insertion of aquaporin-2 channels in the luminal membrane of renal principal cells is essential for urine concentration. Lithium affects urine concentrating ability, and approximately 20% of patients treated with lithium develop nephrogenic diabetes insipidus (NDI), a disorder characterized by polyuria and polydipsia. Lithium-induced NDI is caused by aquaporin-2 downregulation and a reduced ratio of principal/intercalated cells, yet lithium induces principal cell proliferation. Here, we studied how lithium-induced principal cell proliferation can lead to a reduced ratio of principal/intercalated cells using two-dimensional and three-dimensional polarized cultures of mouse renal collecting duct cells and mice treated with clinically relevant lithium concentrations. DNA image cytometry and immunoblotting revealed that lithium initiated proliferation of mouse renal collecting duct cells but also increased the G2/S ratio, indicating G2/M phase arrest. In mice, treatment with lithium for 4, 7, 10, or 13 days led to features of NDI and an increase in the number of principal cells expressing PCNA in the papilla. Remarkably, 30%-40% of the PCNA-positive principal cells also expressed pHistone-H3, a late G2/M phase marker detected in approximately 20% of cells during undisturbed proliferation. Our data reveal that lithium treatment initiates proliferation of renal principal cells but that a significant percentage of these cells are arrested in the late G2 phase, which explains the reduced principal/intercalated cell ratio and may identify the molecular pathway underlying the development of lithium-induced renal fibrosis.
Figures
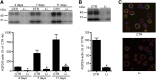


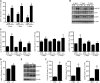

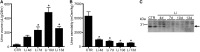

Comment in
-
Lithium in kidney diseases: big roles for the smallest metal.J Am Soc Nephrol. 2014 Mar;25(3):421-3. doi: 10.1681/ASN.2013111216. Epub 2014 Jan 9. J Am Soc Nephrol. 2014. PMID: 24408870 Free PMC article. No abstract available.
-
Basic research: the long and the short of it-the temporal effects of renal lithium exposure are beginning to be unravelled.Nat Rev Nephrol. 2014 Mar;10(3):123. doi: 10.1038/nrneph.2014.10. Epub 2014 Jan 28. Nat Rev Nephrol. 2014. PMID: 24468767 No abstract available.
References
-
- Timmer RT, Sands JM: Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999 - PubMed
-
- Walker RJ, Weggery S, Bedford JJ, McDonald FJ, Ellis G, Leader JP: Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int 67: 291–294, 2005 - PubMed
-
- Bendz H, Aurell M: Drug-induced diabetes insipidus: Incidence, prevention and management. Drug Saf 21: 449–456, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

