The proximal tubule and albuminuria: really!
- PMID: 24408874
- PMCID: PMC3935594
- DOI: 10.1681/ASN.2013090950
The proximal tubule and albuminuria: really!
Abstract
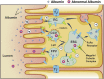
Recent data highlight the role of the proximal tubule (PT) in reabsorbing, processing, and transcytosing urinary albumin from the glomerular filtrate. Innovative techniques and approaches have provided exciting insights into these processes, and numerous investigators have shown that selective PT cell defects lead to significant albuminuria, even reaching nephrotic range in animal models. Thus, the mechanisms of albumin reabsorption and transcytosis are undergoing intense study. Working in concert with megalin and cubilin, a nonselective multireceptor complex that predominantly directs proteins for lysosomal degradation, the neonatal Fc receptor (FcRn) located at the brush border of the apical membrane has been implicated as the "receptor" mediating albumin transcytosis. The FcRn pathway facilitates reabsorption and mediates transcytosis by its pH-dependent binding affinity in endosomal compartments. This also allows for selective albumin sorting within the PT cell. This reclamation pathway minimizes urinary losses and catabolism of albumin, thus prolonging its serum half-life. It may also serve as a molecular sorter to preserve and reclaim normal albumin while allowing "altered" albumin to be catabolized via lysosomal pathways. Here, we critically review the data supporting this novel mechanism.
Keywords: FcRn; cubulin; endocytosis; megalin; nephrotic syndrome; transcytosis.
Figures
References
-
- He XM, Carter DC: Atomic structure and chemistry of human serum albumin. Nature 358: 209–215, 1992 - PubMed
-
- Bhattacharya AA, Grüne T, Curry S: Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303: 721–732, 2000 - PubMed
-
- Kragh-Hansen U: Molecular aspects of ligand binding to serum albumin. Pharmacol Rev 33: 17–53, 1981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

