Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex
- PMID: 24409095
- PMCID: PMC3883644
- DOI: 10.1371/journal.pbio.1001747
Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex
Erratum in
- PLoS Biol. 2014 Apr;12(4):e1001857
Abstract
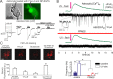
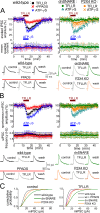
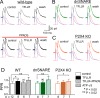
Communication between neuronal and glial cells is important for many brain functions. Astrocytes can modulate synaptic strength via Ca(2+)-stimulated release of various gliotransmitters, including glutamate and ATP. A physiological role of ATP release from astrocytes was suggested by its contribution to glial Ca(2+)-waves and purinergic modulation of neuronal activity and sleep homeostasis. The mechanisms underlying release of gliotransmitters remain uncertain, and exocytosis is the most intriguing and debated pathway. We investigated release of ATP from acutely dissociated cortical astrocytes using "sniff-cell" approach and demonstrated that release is vesicular in nature and can be triggered by elevation of intracellular Ca(2+) via metabotropic and ionotropic receptors or direct UV-uncaging. The exocytosis of ATP from neocortical astrocytes occurred in the millisecond time scale contrasting with much slower nonvesicular release of gliotransmitters via Best1 and TREK-1 channels, reported recently in hippocampus. Furthermore, we discovered that elevation of cytosolic Ca(2+) in cortical astrocytes triggered the release of ATP that directly activated quantal purinergic currents in the pyramidal neurons. The glia-driven burst of purinergic currents in neurons was followed by significant attenuation of both synaptic and tonic inhibition. The Ca(2+)-entry through the neuronal P2X purinoreceptors led to phosphorylation-dependent down-regulation of GABAA receptors. The negative purinergic modulation of postsynaptic GABA receptors was accompanied by small presynaptic enhancement of GABA release. Glia-driven purinergic modulation of inhibitory transmission was not observed in neurons when astrocytes expressed dn-SNARE to impair exocytosis. The astrocyte-driven purinergic currents and glia-driven modulation of GABA receptors were significantly reduced in the P2X4 KO mice. Our data provide a key evidence to support the physiological importance of exocytosis of ATP from astrocytes in the neocortex.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Exocytosis of gliotransmitters from cortical astrocytes: implications for synaptic plasticity and aging.Biochem Soc Trans. 2014 Oct;42(5):1275-81. doi: 10.1042/BST20140163. Biochem Soc Trans. 2014. PMID: 25233403
-
Cannabinoid receptors contribute to astroglial Ca²⁺-signalling and control of synaptic plasticity in the neocortex.Philos Trans R Soc Lond B Biol Sci. 2014 Oct 19;369(1654):20140077. doi: 10.1098/rstb.2014.0077. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25225106 Free PMC article.
-
Stretch-induced Ca2+ independent ATP release in hippocampal astrocytes.J Physiol. 2018 May 15;596(10):1931-1947. doi: 10.1113/JP275805. Epub 2018 Apr 6. J Physiol. 2018. PMID: 29488635 Free PMC article.
-
Ionotropic ATP receptors in neuronal-glial communication.Semin Cell Dev Biol. 2011 Apr;22(2):220-8. doi: 10.1016/j.semcdb.2011.02.012. Epub 2011 Feb 12. Semin Cell Dev Biol. 2011. PMID: 21320623 Review.
-
Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP.Novartis Found Symp. 2006;276:208-17; discussion 217-21, 233-7, 275-81. doi: 10.1002/9780470032244.ch16. Novartis Found Symp. 2006. PMID: 16805432 Review.
Cited by
-
ADORA1-driven brain-sympathetic neuro-adipose connections control body weight and adipose lipid metabolism.Mol Psychiatry. 2021 Jul;26(7):2805-2819. doi: 10.1038/s41380-020-00908-y. Epub 2020 Oct 16. Mol Psychiatry. 2021. PMID: 33067580 Free PMC article.
-
Impact of Autophagy Impairment on Experience- and Diet-Related Synaptic Plasticity.Int J Mol Sci. 2022 Aug 17;23(16):9228. doi: 10.3390/ijms23169228. Int J Mol Sci. 2022. PMID: 36012495 Free PMC article.
-
Astrocytes contribute to pain gating in the spinal cord.Sci Adv. 2021 Nov 5;7(45):eabi6287. doi: 10.1126/sciadv.abi6287. Epub 2021 Nov 3. Sci Adv. 2021. PMID: 34730998 Free PMC article.
-
From Youthful Vigor to Aging Decline: Unravelling the Intrinsic and Extrinsic Determinants of Hippocampal Neural Stem Cell Aging.Cells. 2023 Aug 17;12(16):2086. doi: 10.3390/cells12162086. Cells. 2023. PMID: 37626896 Free PMC article. Review.
-
What do we know about gliotransmitter release from astrocytes?Philos Trans R Soc Lond B Biol Sci. 2014 Oct 19;369(1654):20130592. doi: 10.1098/rstb.2013.0592. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25225086 Free PMC article. Review.
References
-
- Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797. - PubMed
-
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H (2009) Purinergic signalling in the nervous system: an overview. Trends Neurosci 32: 19–29. - PubMed
-
- Lalo U, Pankratov Y, Parpura V, Verkhratsky A (2010) Ionotropic receptors in neuronal-astroglial signalling: what is the role of “excitable” molecules in non-excitable cells. Biochim Biophys Acta 1813: 992–1002. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

