Opposite effects of a high-fat diet and calorie restriction on ciliary neurotrophic factor signaling in the mouse hypothalamus
- PMID: 24409114
- PMCID: PMC3873503
- DOI: 10.3389/fnins.2013.00263
Opposite effects of a high-fat diet and calorie restriction on ciliary neurotrophic factor signaling in the mouse hypothalamus
Abstract
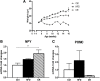
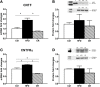
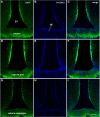
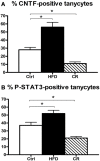
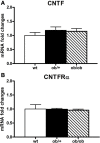
In the mouse hypothalamus, ciliary neurotrophic factor (CNTF) is mainly expressed by ependymal cells and tanycytes of the ependymal layer covering the third ventricle. Since exogenously administered CNTF causes reduced food intake and weight loss, we tested whether endogenous CNTF might be involved in energy balance regulation. We thus evaluated CNTF production and responsiveness in the hypothalamus of mice fed a high-fat diet (HFD), of ob/ob obese mice, and of mice fed a calorie restriction (CR) regimen. RT-PCR showed that CNTF mRNA increased significantly in HFD mice and decreased significantly in CR animals. Western blotting confirmed that CNTF expression was higher in HFD mice and reduced in CR mice, but high interindividual variability blunted the significance of these differences. By immunohistochemistry, hypothalamic tuberal and mammillary region tanycytes stained strongly for CNTF in HFD mice, whereas CR mice exhibited markedly reduced staining. RT-PCR and Western blotting disclosed that changes in CNTF expression were paralleled by changes in the expression of its specific receptor, CNTF receptor α (CNTFRα). Injection of recombinant CNTF and detection of phospho-signal transducer and activator of transcription 3 (P-STAT3) showed that CNTF responsiveness by the ependymal layer, mainly by tanycytes, was higher in HFD than CR mice. In addition, in HFD mice CNTF administration induced distinctive STAT3 signaling in a large neuron population located in the dorsomedial and ventromedial nuclei, perifornical area and mammillary body. The hypothalamic expression of CNTF and CNTFRα did not change in the hyperphagic, leptin-deficient ob/ob obese mice; accordingly, P-STAT3 immunoreactivity in CNTF-treated ob/ob mice was confined to ependymal layer and arcuate neurons. Collectively, these data suggest that hypothalamic CNTF is involved in controlling the energy balance and that CNTF signaling plays a role in HFD obese mice at specific sites.
Keywords: CNTF receptor; STAT3; ependyma; fasting; median eminence; obesity; tanycytes; third ventricle.
Figures


Similar articles
-
Ciliary Neurotrophic Factor Acts on Distinctive Hypothalamic Arcuate Neurons and Promotes Leptin Entry Into and Action on the Mouse Hypothalamus.Front Cell Neurosci. 2020 May 21;14:140. doi: 10.3389/fncel.2020.00140. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32528252 Free PMC article.
-
Constitutive expression of ciliary neurotrophic factor in mouse hypothalamus.J Anat. 2012 Jun;220(6):622-31. doi: 10.1111/j.1469-7580.2012.01498.x. Epub 2012 Mar 28. J Anat. 2012. PMID: 22458546 Free PMC article.
-
Activation of transcription factors STAT1 and STAT5 in the mouse median eminence after systemic ciliary neurotrophic factor administration.Brain Res. 2015 Oct 5;1622:217-29. doi: 10.1016/j.brainres.2015.06.028. Epub 2015 Jun 30. Brain Res. 2015. PMID: 26133794
-
The ciliary neurotrophic factor and its receptor, CNTFR alpha.Pharm Acta Helv. 2000 Mar;74(2-3):265-72. doi: 10.1016/s0031-6865(99)00050-3. Pharm Acta Helv. 2000. PMID: 10812968 Review.
-
Hypothalamic tanycytes: potential roles in the control of feeding and energy balance.Trends Neurosci. 2013 Feb;36(2):91-100. doi: 10.1016/j.tins.2012.12.008. Epub 2013 Jan 17. Trends Neurosci. 2013. PMID: 23332797 Free PMC article. Review.
Cited by
-
Action of Administered Ciliary Neurotrophic Factor on the Mouse Dorsal Vagal Complex.Front Neurosci. 2016 Jun 27;10:289. doi: 10.3389/fnins.2016.00289. eCollection 2016. Front Neurosci. 2016. PMID: 27445662 Free PMC article.
-
Potential role of tanycyte-derived neurogenesis in Alzheimer's disease.Neural Regen Res. 2025 Jun 1;20(6):1599-1612. doi: 10.4103/NRR.NRR-D-23-01865. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934388 Free PMC article.
-
Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice.Front Neurosci. 2014 Jun 13;8:157. doi: 10.3389/fnins.2014.00157. eCollection 2014. Front Neurosci. 2014. PMID: 24982613 Free PMC article.
-
Ciliary Neurotrophic Factor Acts on Distinctive Hypothalamic Arcuate Neurons and Promotes Leptin Entry Into and Action on the Mouse Hypothalamus.Front Cell Neurosci. 2020 May 21;14:140. doi: 10.3389/fncel.2020.00140. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32528252 Free PMC article.
-
Ciliary neurotrophic factor is increased in the plasma of patients with obesity and its levels correlate with diabetes and inflammation indices.Sci Rep. 2022 May 18;12(1):8331. doi: 10.1038/s41598-022-11942-x. Sci Rep. 2022. PMID: 35585213 Free PMC article.
References
-
- ACTS. (1996). A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology 46, 1244–1249 - PubMed
-
- Blüher S., Moschos S., Bullen J., Jr., Kokkotou E., Maratos-Flier E., Wiegand S. J., et al. (2004). Ciliary neurotrophic factorAx15 alters energy homeostasis, decreases body weight, and improves metabolic control in diet-induced obese and UCP1-DTA mice. Diabetes 53, 2787–2796 10.2337/diabetes.53.11.2787 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

