Transcranial slow oscillation stimulation during NREM sleep enhances acquisition of the radial maze task and modulates cortical network activity in rats
- PMID: 24409131
- PMCID: PMC3884143
- DOI: 10.3389/fnbeh.2013.00220
Transcranial slow oscillation stimulation during NREM sleep enhances acquisition of the radial maze task and modulates cortical network activity in rats
Abstract
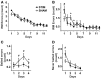
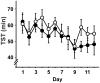
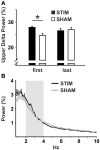
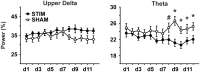
Slow wave sleep, hallmarked by the occurrence of slow oscillations (SO), plays an important role for the consolidation of hippocampus-dependent memories. Transcranial stimulation by weak electric currents oscillating at the endogenous SO frequency (SO-tDCS) during post-learning sleep was previously shown by us to boost SO activity and improve the consolidation of hippocampus-dependent memory in human subjects. Here, we aimed at replicating and extending these results to a rodent model. Rats were trained for 12 days at the beginning of their inactive phase in the reference memory version of the radial arm maze. In a between subjects design, animals received SO-tDCS over prefrontal cortex (PFC) or sham stimulation within a time frame of 1 h during subsequent non-rapid eye movement (NREM) sleep. Applied over multiple daily sessions SO-tDCS impacted cortical network activity as measured by EEG and behavior: at the EEG level, SO-tDCS enhanced post-stimulation upper delta (2-4 Hz) activity whereby the first stimulations of each day were preferentially affected. Furthermore, commencing on day 8, SO-tDCS acutely decreased theta activity indicating long-term effects on cortical networks. Behaviorally, working memory for baited maze arms was enhanced up to day 4, indicating enhanced consolidation of task-inherent rules, while reference memory errors did not differ between groups. Taken together, we could show here for the first time an effect of SO-tDCS during NREM sleep on cognitive functions and on cortical activity in a rodent model.
Keywords: EEG; consolidation; reference memory; sleep; slow oscillation stimulation; tDCS; working memory.
Figures
Similar articles
-
Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats.Brain Stimul. 2014 Jul-Aug;7(4):508-15. doi: 10.1016/j.brs.2014.03.001. Epub 2014 Mar 12. Brain Stimul. 2014. PMID: 24698973
-
Boosting Slow Oscillatory Activity Using tDCS during Early Nocturnal Slow Wave Sleep Does Not Improve Memory Consolidation in Healthy Older Adults.Brain Stimul. 2016 Sep-Oct;9(5):730-739. doi: 10.1016/j.brs.2016.04.016. Epub 2016 Apr 28. Brain Stimul. 2016. PMID: 27247261 Clinical Trial.
-
Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans.PLoS One. 2011 Feb 14;6(2):e16905. doi: 10.1371/journal.pone.0016905. PLoS One. 2011. PMID: 21340034 Free PMC article. Clinical Trial.
-
Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment.J Neurosci. 2017 Jul 26;37(30):7111-7124. doi: 10.1523/JNEUROSCI.0260-17.2017. Epub 2017 Jun 21. J Neurosci. 2017. PMID: 28637840 Free PMC article. Review.
-
Contribution of transcranial oscillatory stimulation to research on neural networks: an emphasis on hippocampo-neocortical rhythms.Front Hum Neurosci. 2013 Sep 26;7:614. doi: 10.3389/fnhum.2013.00614. Front Hum Neurosci. 2013. PMID: 24133431 Free PMC article. Review.
Cited by
-
Somatostatin+/nNOS+ neurons are involved in delta electroencephalogram activity and cortical-dependent recognition memory.Sleep. 2019 Oct 9;42(10):zsz143. doi: 10.1093/sleep/zsz143. Sleep. 2019. PMID: 31328777 Free PMC article.
-
Neonatal Isoflurane Does Not Affect Sleep Architecture and Minimally Alters Neuronal Beta Oscillations in Adolescent Rats.Front Behav Neurosci. 2021 Nov 1;15:703859. doi: 10.3389/fnbeh.2021.703859. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34790103 Free PMC article.
-
Neurobiological correlates of state-dependent context fear.Learn Mem. 2017 Aug 16;24(9):385-391. doi: 10.1101/lm.045542.117. Print 2017 Sep. Learn Mem. 2017. PMID: 28814463 Free PMC article.
-
Sleep-dependent memory consolidation and its implications for psychiatry.J Neural Transm (Vienna). 2017 Feb;124(Suppl 1):163-178. doi: 10.1007/s00702-015-1476-3. Epub 2015 Oct 30. J Neural Transm (Vienna). 2017. PMID: 26518213 Review.
-
Levels of Interference in Long and Short-Term Memory Differentially Modulate Non-REM and REM Sleep.Sleep. 2016 Dec 1;39(12):2173-2188. doi: 10.5665/sleep.6322. Sleep. 2016. PMID: 27748246 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

