Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse
- PMID: 24409148
- PMCID: PMC3884214
- DOI: 10.3389/fphar.2013.00172
Convergence of dopamine and glutamate signaling onto striatal ERK activation in response to drugs of abuse
Abstract
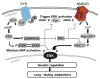
Despite their distinct targets, all addictive drugs commonly abused by humans evoke increases in dopamine (DA) concentration within the striatum. The main DA Guanine nucleotide binding protein couple receptors (GPCRs) expressed by medium-sized spiny neurons of the striatum are the D1R and D2R, which are positively and negatively coupled to cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling, respectively. These two DA GPCRs are largely segregated into distinct neuronal populations, where they are co-expressed with glutamate receptors in dendritic spines. Direct and indirect interactions between DA GPCRs and glutamate receptors are the molecular basis by which DA modulates glutamate transmission and controls striatal plasticity and behavior induced by drugs of abuse. A major downstream target of striatal D1R is the extracellular signal-regulated kinase (ERK) kinase pathway. ERK activation by drugs of abuse behaves as a key integrator of D1R and glutamate NMDAR signaling. Once activated, ERK can trigger chromatin remodeling and induce gene expression that permits long-term cellular alterations and drug-induced morphological and behavioral changes. Besides the classical cAMP/PKA pathway, downstream of D1R, recent evidence implicates a cAMP-independent crosstalk mechanism by which the D1R potentiates NMDAR-mediated calcium influx and ERK activation. The mounting evidence of reciprocal modulation of DA and glutamate receptors adds further intricacy to striatal synaptic signaling and is liable to prove relevant for addictive drug-induced signaling, plasticity, and behavior. Herein, we review the evidence that built our understanding of the consequences of this synergistic signaling for the actions of drugs of abuse.
Keywords: ERK; GPCR; addiction; crosstalk; dopamine; receptors; signaling; striatum.
Figures
Similar articles
-
Segregation and crosstalk of D1 receptor-mediated activation of ERK in striatal medium spiny neurons upon acute administration of psychostimulants.PLoS Comput Biol. 2014 Jan 30;10(1):e1003445. doi: 10.1371/journal.pcbi.1003445. eCollection 2014 Jan. PLoS Comput Biol. 2014. PMID: 24499932 Free PMC article.
-
D1R/GluN1 complexes in the striatum integrate dopamine and glutamate signalling to control synaptic plasticity and cocaine-induced responses.Mol Psychiatry. 2014 Dec;19(12):1295-304. doi: 10.1038/mp.2014.73. Epub 2014 Jul 29. Mol Psychiatry. 2014. PMID: 25070539 Free PMC article.
-
Sensing Positive versus Negative Reward Signals through Adenylyl Cyclase-Coupled GPCRs in Direct and Indirect Pathway Striatal Medium Spiny Neurons.J Neurosci. 2015 Oct 14;35(41):14017-30. doi: 10.1523/JNEUROSCI.0730-15.2015. J Neurosci. 2015. PMID: 26468202 Free PMC article.
-
Cell-Type-Specific Adaptions in Striatal Medium-Sized Spiny Neurons and Their Roles in Behavioral Responses to Drugs of Abuse.Front Synaptic Neurosci. 2021 Dec 14;13:799274. doi: 10.3389/fnsyn.2021.799274. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34970134 Free PMC article. Review.
-
Brain Dopamine Transmission in Health and Parkinson's Disease: Modulation of Synaptic Transmission and Plasticity Through Volume Transmission and Dopamine Heteroreceptors.Front Synaptic Neurosci. 2018 Jul 10;10:20. doi: 10.3389/fnsyn.2018.00020. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30042672 Free PMC article. Review.
Cited by
-
Selective remodeling of glutamatergic transmission to striatal cholinergic interneurons after dopamine depletion.Eur J Neurosci. 2019 Mar;49(6):824-833. doi: 10.1111/ejn.13715. Epub 2017 Oct 6. Eur J Neurosci. 2019. PMID: 28922504 Free PMC article.
-
Adaptations in glutathione-based redox protein signaling pathways and alcohol drinking across species.Biomed Pharmacother. 2024 Nov;180:117514. doi: 10.1016/j.biopha.2024.117514. Epub 2024 Oct 2. Biomed Pharmacother. 2024. PMID: 39362067 Free PMC article.
-
Dopamine D1 and Glutamate Receptors Co-operate With Brain-Derived Neurotrophic Factor (BDNF) and TrkB to Modulate ERK Signaling in Adult Striatal Slices.Front Cell Neurosci. 2020 Nov 16;14:564106. doi: 10.3389/fncel.2020.564106. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33304241 Free PMC article.
-
A Rational Design of α-Helix-Shaped Peptides Employing the Hydrogen-Bond Surrogate Approach: A Modulation Strategy for Ras-RasGRF1 Interaction in Neuropsychiatric Disorders.Pharmaceuticals (Basel). 2021 Oct 28;14(11):1099. doi: 10.3390/ph14111099. Pharmaceuticals (Basel). 2021. PMID: 34832880 Free PMC article.
-
Glutamate Afferents From the Medial Prefrontal Cortex Mediate Nucleus Accumbens Activation by Female Sexual Behavior.Front Behav Neurosci. 2019 Oct 4;13:227. doi: 10.3389/fnbeh.2019.00227. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31636548 Free PMC article.
References
-
- Bertran-Gonzalez J., Bosch C., Maroteaux M., Matamales M., Hervé D., Valjent E., et al. (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28 5671–5685 10.1523/JNEUROSCI.1039-08.2008 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

