Phosphoinositide regulation of inward rectifier potassium (Kir) channels
- PMID: 24409153
- PMCID: PMC3884141
- DOI: 10.3389/fphys.2013.00404
Phosphoinositide regulation of inward rectifier potassium (Kir) channels
Abstract
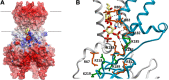
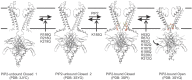
Inward rectifier potassium (Kir) channels are integral membrane proteins charged with a key role in establishing the resting membrane potential of excitable cells through selective control of the permeation of K(+) ions across cell membranes. In conjunction with secondary anionic phospholipids, members of this family are directly regulated by phosphoinositides (PIPs) in the absence of other proteins or downstream signaling pathways. Different Kir isoforms display distinct specificities for the activating PIPs but all eukaryotic Kir channels are activated by PI(4,5)P2. On the other hand, the bacterial KirBac1.1 channel is inhibited by PIPs. Recent crystal structures of eukaryotic Kir channels in apo and lipid bound forms reveal one specific binding site per subunit, formed at the interface of N- and C-terminal domains, just beyond the transmembrane segments and clearly involving some of the key residues previously identified as controlling PI(4,5)P2 sensitivity. Computational, biochemical, and biophysical approaches have attempted to address the energetic determinants of PIP binding and selectivity among Kir channel isoforms, as well as the conformational changes that trigger channel gating. Here we review our current understanding of the molecular determinants of PIP regulation of Kir channel activity, including in context with other lipid modulators, and provide further discussion on the key questions that remain to be answered.
Keywords: Kir channel; inward rectifier potassium channels; ion channel; ion channel gating; ligand-gated ion channels; lipid protein interactions; phosphoinositides.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

