Nuclear Factor κB is Required for Tumor Growth Inhibition Mediated by Enavatuzumab (PDL192), a Humanized Monoclonal Antibody to TweakR
- PMID: 24409185
- PMCID: PMC3884146
- DOI: 10.3389/fimmu.2013.00505
Nuclear Factor κB is Required for Tumor Growth Inhibition Mediated by Enavatuzumab (PDL192), a Humanized Monoclonal Antibody to TweakR
Abstract
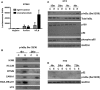
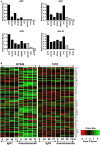
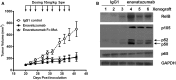
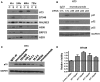
TweakR is a TNF receptor family member, whose natural ligand is the multifunctional cytokine TWEAK. The growth inhibitory activity observed following TweakR stimulation in certain cancer cell lines and the overexpression of TweakR in many solid tumor types led to the development of enavatuzumab (PDL192), a humanized IgG1 monoclonal antibody to TweakR. The purpose of this study was to determine the mechanism of action of enavatuzumab's tumor growth inhibition and to provide insight into the biology behind TweakR as a cancer therapeutic target. A panel of 105 cancer lines was treated with enavatuzumab in vitro; and 29 cell lines of varying solid tumor backgrounds had >25% growth inhibition in response to the antibody. Treatment of sensitive cell lines with enavatuzumab resulted in the in vitro and in vivo (xenograft) activation of both classical (p50, p65) and non-classical (p52, RelB) NFκB pathways. Using NFκB DNA binding functional ELISAs and microarray analysis, we observed increased activation of NFκB subunits and NFκB-regulated genes in sensitive cells over that observed in resistant cell lines. Inhibiting NFκB subunits (p50, p65, RelB, p52) and upstream kinases (IKK1, IKK2) with siRNA and chemical inhibitors consistently blocked enavatuzumab's activity. Furthermore, enavatuzumab treatment resulted in NFκB-dependent reduction in cell division as seen by the activation of the cell cycle inhibitor p21 both in vitro and in vivo. The finding that NFκB drives the growth inhibitory activity of enavatuzumab suggests that targeting TweakR with enavatuzumab may represent a novel cancer treatment strategy.
Keywords: Fn14; NFκB; TweakR; enavatuzumab; monoclonal antibody; p21.
Figures



Similar articles
-
Expression of TweakR in breast cancer and preclinical activity of enavatuzumab, a humanized anti-TweakR mAb.J Cancer Res Clin Oncol. 2013 Feb;139(2):315-25. doi: 10.1007/s00432-012-1332-x. Epub 2012 Oct 17. J Cancer Res Clin Oncol. 2013. PMID: 23073510 Free PMC article.
-
Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms.Clin Cancer Res. 2010 Jan 15;16(2):497-508. doi: 10.1158/1078-0432.CCR-09-1929. Epub 2010 Jan 12. Clin Cancer Res. 2010. PMID: 20068083
-
Predictive gene signature of response to the anti-TweakR mAb PDL192 in patient-derived breast cancer xenografts.PLoS One. 2014 Nov 6;9(11):e104227. doi: 10.1371/journal.pone.0104227. eCollection 2014. PLoS One. 2014. PMID: 25375638 Free PMC article.
-
TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor.Cytokine Growth Factor Rev. 2003 Jun-Aug;14(3-4):241-9. doi: 10.1016/s1359-6101(03)00019-4. Cytokine Growth Factor Rev. 2003. PMID: 12787562 Review.
-
The TWEAK-Fn14 system as a potential drug target.Br J Pharmacol. 2013 Oct;170(4):748-64. doi: 10.1111/bph.12337. Br J Pharmacol. 2013. PMID: 23957828 Free PMC article. Review.
Cited by
-
Expression of TweakR in breast cancer and preclinical activity of enavatuzumab, a humanized anti-TweakR mAb.J Cancer Res Clin Oncol. 2013 Feb;139(2):315-25. doi: 10.1007/s00432-012-1332-x. Epub 2012 Oct 17. J Cancer Res Clin Oncol. 2013. PMID: 23073510 Free PMC article.
-
Targeting fibroblast growth factor (FGF)-inducible 14 (Fn14) for tumor therapy.Front Pharmacol. 2022 Oct 21;13:935086. doi: 10.3389/fphar.2022.935086. eCollection 2022. Front Pharmacol. 2022. PMID: 36339601 Free PMC article. Review.
-
Antibody-based soluble and membrane-bound TWEAK mimicking agonists with FcγR-independent activity.Front Immunol. 2023 Jul 11;14:1194610. doi: 10.3389/fimmu.2023.1194610. eCollection 2023. Front Immunol. 2023. PMID: 37545514 Free PMC article.
-
Enavatuzumab, a Humanized Anti-TWEAK Receptor Monoclonal Antibody, Exerts Antitumor Activity through Attracting and Activating Innate Immune Effector Cells.J Immunol Res. 2017;2017:5737159. doi: 10.1155/2017/5737159. Epub 2017 Sep 17. J Immunol Res. 2017. PMID: 29075649 Free PMC article.
-
TWEAK activation of the non-canonical NF-κB signaling pathway differentially regulates melanoma and prostate cancer cell invasion.Oncotarget. 2016 Dec 6;7(49):81474-81492. doi: 10.18632/oncotarget.13034. Oncotarget. 2016. PMID: 27821799 Free PMC article.
References
-
- Jakubowski A, Browning B, Lukashev M, Sizing I, Thompson JS, Benjamin CD, et al. Dual role for TWEAK in angiogenic regulation. J Cell Sci (2002) 115:267–74 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

