Regulating intestinal function to reduce atherogenic lipoproteins
- PMID: 24409204
- PMCID: PMC3881294
- DOI: 10.2217/clp.13.40
Regulating intestinal function to reduce atherogenic lipoproteins
Abstract
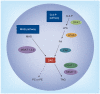
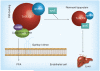
Significant knowledge regarding different molecules involved in the transport of dietary fat into the circulation has been garnered. Studies point to the possibility that accumulation of intestine-derived lipoproteins in the plasma could contribute to atherosclerosis. This article provides a brief overview of dietary lipid metabolism and studies in mice supporting the hypothesis that intestinal lipoproteins contribute to atherosclerosis. Deficiencies in lipoprotein lipase and Gpihbp1, and overexpression of heparanse in mice, are associated with increases in atherosclerosis, suggesting that defects in catabolism of larger lipoproteins in the plasma contribute to atherosclerosis. Furthermore, inositol-requiring enzyme 1β-deficient mice that produce more intestinal lipoproteins also develop more atherosclerosis. Thus, increases in plasma intestinal lipoproteins due to either overproduction or reduced catabolism result in augmented atherosclerosis. Intestinal lipoproteins tend to adhere strongly to subendothelial proteoglycans, elicit an inflammatory response by endothelial cells and activate macrophages, contributing to the initiation and progression of the disease. Thus, molecules that reduce intestinal lipid absorption can be useful in lowering atherosclerosis.
Keywords: MTP; apoB; atherosclerosis; cholesterol; chylomicronemia; hyperlipidemia; hypertriglyceridemia; lipoproteins; triglyceride.
Figures

Similar articles
-
ABCA1 overexpression in the liver of LDLr-KO mice leads to accumulation of pro-atherogenic lipoproteins and enhanced atherosclerosis.J Biol Chem. 2006 Nov 3;281(44):33053-65. doi: 10.1074/jbc.M604526200. Epub 2006 Aug 23. J Biol Chem. 2006. PMID: 16928680
-
Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited.J Lipid Res. 2007 Jan;48(1):114-26. doi: 10.1194/jlr.M600353-JLR200. Epub 2006 Oct 23. J Lipid Res. 2007. PMID: 17060690
-
Postprandial lipoproteins and the molecular regulation of vascular homeostasis.Prog Lipid Res. 2013 Oct;52(4):446-64. doi: 10.1016/j.plipres.2013.06.001. Epub 2013 Jun 15. Prog Lipid Res. 2013. PMID: 23774609 Review.
-
MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion.Nat Med. 2013 Jul;19(7):892-900. doi: 10.1038/nm.3200. Epub 2013 Jun 9. Nat Med. 2013. PMID: 23749231 Free PMC article.
-
Metabolism and Modification of Apolipoprotein B-Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis.Biol Pharm Bull. 2016;39(1):1-24. doi: 10.1248/bpb.b15-00716. Biol Pharm Bull. 2016. PMID: 26725424 Review.
Cited by
-
Resveratrol inhibits lipid accumulation in the intestine of atherosclerotic mice and macrophages.J Cell Mol Med. 2019 Jun;23(6):4313-4325. doi: 10.1111/jcmm.14323. Epub 2019 Apr 7. J Cell Mol Med. 2019. PMID: 30957417 Free PMC article.
-
Lipid transfer proteins in the assembly of apoB-containing lipoproteins.J Lipid Res. 2018 Jul;59(7):1094-1102. doi: 10.1194/jlr.R083451. Epub 2018 Apr 12. J Lipid Res. 2018. PMID: 29650752 Free PMC article. Review.
-
Intestinal lipid absorption and lipoprotein formation.Curr Opin Lipidol. 2014 Jun;25(3):200-6. doi: 10.1097/MOL.0000000000000084. Curr Opin Lipidol. 2014. PMID: 24751933 Free PMC article. Review.
-
Circadian regulators of intestinal lipid absorption.J Lipid Res. 2015 Apr;56(4):761-70. doi: 10.1194/jlr.R051573. Epub 2014 Jul 23. J Lipid Res. 2015. PMID: 25057097 Free PMC article. Review.
-
HEWL interacts with dissipated oleic acid micelles, and decreases oleic acid cytotoxicity.PLoS One. 2019 Feb 22;14(2):e0212648. doi: 10.1371/journal.pone.0212648. eCollection 2019. PLoS One. 2019. PMID: 30794655 Free PMC article.
References
-
- Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. - PubMed
-
- Lairon D. Macronutrient intake and modulation on chylomicron production and clearance. Atheroscler. Suppl. 2008;9:45–48. - PubMed
-
- Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J. Intern. Med. 1999;246:341–355. - PubMed
-
- Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br. J. Nutr. 2007;98:458–473. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
