TRIP8b is required for maximal expression of HCN1 in the mouse retina
- PMID: 24409334
- PMCID: PMC3883711
- DOI: 10.1371/journal.pone.0085850
TRIP8b is required for maximal expression of HCN1 in the mouse retina
Abstract
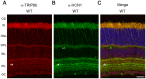
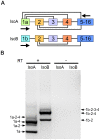
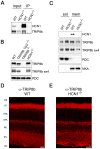
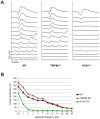
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are cation-selective channels present in retina, brain and heart. The activity of HCN channels contributes to signal integration, cell excitability and pacemaker activity. HCN1 channels expressed in photoreceptors participate in keeping light responses transient and are required for normal mesopic vision. The subcellular localization of HCN1 varies among cell types. In photoreceptors HCN1 is concentrated in the inner segments while in other retinal neurons, HCN1 is evenly distributed though the cell. This is in contrast to hippocampal neurons where HCN1 is concentrated in a subset of dendrites. A key regulator of HCN1 trafficking and activity is tetratricopeptide repeat-containing Rab8b interacting protein (TRIP8b). Multiple splice isoforms of TRIP8b are expressed throughout the brain and can differentially regulate the surface expression and activity of HCN1. The purpose of the present study was to determine which isoforms of TRIP8b are expressed in the retina and to test if loss of TRIP8b alters HCN1 expression or trafficking. We found that TRIP8b colocalizes with HCN1 in multiple retina neurons and all major splice isoforms of TRIP8b are expressed in the retina. Photoreceptors express three different isoforms. In TRIP8b knockout mice, the ability of HCN1 to traffic to the surface of retinal neurons is unaffected. However, there is a large decrease in the total amount of HCN1. We conclude that TRIP8b in the retina is needed to achieve maximal expression of HCN1.
Conflict of interest statement
Figures



References
-
- Fain GL, Quandt FN, Bastian BL, Gerschenfeld HM (1978) Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature 272: 466–469. - PubMed
-
- Knop GC, Seeliger MW, Thiel F, Mataruga A, Kaupp UB, et al. (2008) Light responses in the mouse retina are prolonged upon targeted deletion of the HCN1 channel gene. Eur J Neurosci 28: 2221–2230. - PubMed
-
- Seeliger MW, Brombas A, Weiler R, Humphries P, Knop G, et al. (2011) Modulation of rod photoreceptor output by HCN1 channels is essential for regular mesopic cone vision. Nat Commun 2: 532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

