Role of indacaterol, a once-daily bronchodilator, in chronic obstructive pulmonary disease
- PMID: 24409359
- PMCID: PMC3886694
- DOI: 10.3978/j.issn.2072-1439.2013.10.11
Role of indacaterol, a once-daily bronchodilator, in chronic obstructive pulmonary disease
Abstract
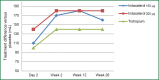
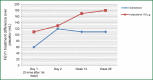
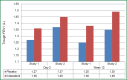
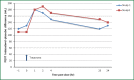
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction that can lead to lung destruction and dyspnea. Although there has been a slight reduction in mortality in recent decades, COPD is still a serious health problem that has enormous costs and utilizes significant medical resources. There have been a number of pharmacologic interventions that have been developed for the treatment of COPD. Current guidelines recommend the use of long-acting bronchodilators for the treatment of moderate and severe stage COPD, since they have been shown to improve lung function, respiratory symptoms, and quality of life. Indacaterol is a once-daily beta2-agonist (β2-agonist) delivered by a single-dose dry powder inhaler used for the treatment of COPD. It is currently approved at a dose of 75 μg in the United States and a dose of 150 μg with a maximal dose of 300 μg in Europe and other countries. Several studies show that indacaterol was statistically superior to both long-acting β2-agonist, formoterol and salmeterol, as well as, noninferior to tiotropium. Indacaterol is generally well tolerated and has a good safety profile. Other studies show that there is an additive bronchodilator response with the addition of indacaterol to tiotropium, which would provide a once-daily treatment option for patient with moderate to severe COPD. This review discusses the pharmacokinetic, comparative efficacy and safety data for indacaterol.
Keywords: Indacaterol; bronchodilator; chronic obstructive pulmonary disease (COPD); formoterol; long-acting beta2-agonist (β2-agonist); salmeterol; tiotropium.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
