Characterization of BU09059: a novel potent selective κ-receptor antagonist
- PMID: 24410326
- PMCID: PMC3963132
- DOI: 10.1021/cn4001507
Characterization of BU09059: a novel potent selective κ-receptor antagonist
Abstract
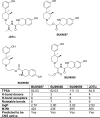
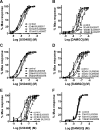
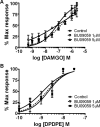
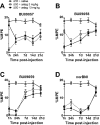
Kappa-opioid receptor (κ) antagonists are potential therapeutic agents for a range of psychiatric disorders. The feasibility of developing κ-antagonists has been limited by the pharmacodynamic properties of prototypic κ-selective antagonists; that is, they inhibit receptor signaling for weeks after a single administration. To address this issue, novel trans-(3R,4R)-dimethyl-4-(3-hydroxyphenyl) piperidine derivatives, based on JDTic, were designed using soft-drug principles. The aim was to determine if the phenylpiperidine-based series of κ-antagonists was amenable to incorporation of a potentially metabolically labile group, while retaining good affinity and selectivity for the κ-receptor. Opioid receptor binding affinity and selectivity of three novel compounds (BU09057, BU09058, and BU09059) were tested. BU09059, which most closely resembles JDTic, had nanomolar affinity for the κ-receptor, with 15-fold and 616-fold selectivity over μ- and δ-receptors, respectively. In isolated tissues, BU09059 was a potent and selective κ-antagonist (pA2 8.62) compared with BU09057 (pA2 6.87) and BU09058 (pA2 6.76) which were not κ-selective. In vivo, BU09059 (3 and 10 mg/kg) significantly blocked U50,488-induced antinociception and was as potent as, but shorter acting than, the prototypic selective κ-antagonist norBNI. These data show that a new JDTic analogue, BU09059, retains high affinity and selectivity for the κ-receptor and has a shorter duration of κ-antagonist action in vivo.
Figures
Similar articles
-
The discovery and development of the N-substituted trans-3,4-dimethyl-4-(3'-hydroxyphenyl)piperidine class of pure opioid receptor antagonists.ChemMedChem. 2014 Aug;9(8):1638-54. doi: 10.1002/cmdc.201402142. Epub 2014 Jun 30. ChemMedChem. 2014. PMID: 24981721 Free PMC article. Review.
-
Identification of the first trans-(3R,4R)- dimethyl-4-(3-hydroxyphenyl)piperidine derivative to possess highly potent and selective opioid kappa receptor antagonist activity.J Med Chem. 2001 Aug 16;44(17):2687-90. doi: 10.1021/jm015521r. J Med Chem. 2001. PMID: 11495579
-
Discovery of the first small-molecule opioid pan antagonist with nanomolar affinity at mu, delta, kappa, and nociceptin opioid receptors.ACS Chem Neurosci. 2015 Apr 15;6(4):646-57. doi: 10.1021/cn500367b. Epub 2015 Feb 18. ACS Chem Neurosci. 2015. PMID: 25635572 Free PMC article.
-
Selective κ opioid antagonists nor-BNI, GNTI and JDTic have low affinities for non-opioid receptors and transporters.PLoS One. 2013 Aug 14;8(8):e70701. doi: 10.1371/journal.pone.0070701. eCollection 2013. PLoS One. 2013. PMID: 23976952 Free PMC article.
-
A review of kappa opioid receptor antagonists and their clinical trial landscape.Eur J Med Chem. 2025 Apr 5;287:117205. doi: 10.1016/j.ejmech.2024.117205. Epub 2024 Dec 25. Eur J Med Chem. 2025. PMID: 39893986 Review.
Cited by
-
Combined administration of buprenorphine and naltrexone produces antidepressant-like effects in mice.J Psychopharmacol. 2015 Jul;29(7):812-21. doi: 10.1177/0269881115586937. Epub 2015 Jun 4. J Psychopharmacol. 2015. PMID: 26045511 Free PMC article.
-
Kappa Opioid Receptor Ligands and Pharmacology: Diphenethylamines, a Class of Structurally Distinct, Selective Kappa Opioid Ligands.Handb Exp Pharmacol. 2022;271:163-195. doi: 10.1007/164_2020_431. Handb Exp Pharmacol. 2022. PMID: 33454858 Review.
-
Targeting opioid receptor signaling in depression: do we need selective κ opioid receptor antagonists?Neuronal Signal. 2018 May 14;2(2):NS20170145. doi: 10.1042/NS20170145. eCollection 2018 Jun. Neuronal Signal. 2018. PMID: 32714584 Free PMC article. Review.
-
The discovery and development of the N-substituted trans-3,4-dimethyl-4-(3'-hydroxyphenyl)piperidine class of pure opioid receptor antagonists.ChemMedChem. 2014 Aug;9(8):1638-54. doi: 10.1002/cmdc.201402142. Epub 2014 Jun 30. ChemMedChem. 2014. PMID: 24981721 Free PMC article. Review.
-
Antidepressant-like effects of BU10119, a novel buprenorphine analogue with mixed κ/μ receptor antagonist properties, in mice.Br J Pharmacol. 2018 Jul;175(14):2869-2880. doi: 10.1111/bph.14060. Epub 2017 Nov 6. Br J Pharmacol. 2018. PMID: 28967123 Free PMC article.
References
-
- Mansour A.; Fox C. A.; Burke S.; Meng F.; Thompson R. C.; Akil H.; Watson S. J. (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol. 350, 412–438. - PubMed
-
- Kitchen I.; Slowe S. J.; Matthes H. W. D.; Kieffer B. (1997) Quantitative autoradiographic mapping of μ-, δ- and κ-opioid receptors in knockout mice lacking the μ-opioid receptor gene. Brain Res. 778, 73–88. - PubMed
-
- Lin S.; Boey D.; Lee N.; Schwarzer C.; Sainsbury A.; Herzog H. (2006) Distribution of prodynorphin mRNA and its interaction with the NPY system in the mouse brain. Neuropeptides 40, 115–123. - PubMed
-
- Pfeiffer A.; Brantl V.; Herz A.; Emrich H. M. (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233, 774–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

