Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation
- PMID: 24410370
- PMCID: PMC4029047
- DOI: 10.1089/ten.TEA.2013.0515
Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation
Abstract
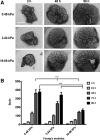
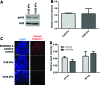
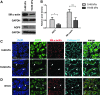
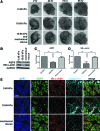
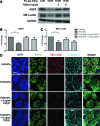
Substrate compliance is reported to alter cell phenotype, but little is known about the effects of compliance on cell development within the context of a complex tissue. In this study, we used 0.48 and 19.66 kPa polyacrylamide gels to test the effects of the substrate modulus on submandibular salivary gland development in culture and found a significant decrease in branching morphogenesis in explants grown on the stiff 19.66 kPa gels relative to those grown on the more physiologically compliant 0.48 kPa gels. While proliferation and apoptosis were not affected by the substrate modulus, tissue architecture and epithelial acinar cell differentiation were profoundly perturbed by aberrant, high stiffness. The glands cultured on 0.48 kPa gels were similar to developing glands in morphology and expression of the differentiation markers smooth muscle alpha-actin (SM α-actin) in developing myoepithelial cells and aquaporin 5 (AQP5) in proacinar cells. At 19.66 kPa, however, tissue morphology and the expression and distribution of SM α-actin and AQP5 were disrupted. Significantly, aberrant gland development at 19.66 kPa could be rescued by both mechanical and chemical stimuli. Transfer of glands from 19.66 to 0.48 kPa gels resulted in substantial recovery of acinar structure and differentiation, and addition of exogenous transforming growth factor beta 1 at 19.66 kPa resulted in a partial rescue of morphology and differentiation within the proacinar buds. These results indicate that environmental compliance is critical for organogenesis, and suggest that both mechanical and chemical stimuli can be exploited to promote organ development in the contexts of tissue engineering and organ regeneration.
Figures
Similar articles
-
TGFβ signaling promotes matrix assembly during mechanosensitive embryonic salivary gland restoration.Matrix Biol. 2015 Apr;43:109-24. doi: 10.1016/j.matbio.2015.01.020. Epub 2015 Jan 31. Matrix Biol. 2015. PMID: 25652203 Free PMC article.
-
Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development.Organogenesis. 2016 Oct;12(4):194-216. doi: 10.1080/15476278.2016.1252887. Epub 2016 Nov 14. Organogenesis. 2016. PMID: 27841695 Free PMC article.
-
FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids.J Cell Sci. 2018 Feb 20;131(4):jcs208728. doi: 10.1242/jcs.208728. J Cell Sci. 2018. PMID: 29361536 Free PMC article.
-
Morphogenesis of normal human salivary gland cells in vitro.Histol Histopathol. 1994 Oct;9(4):781-90. Histol Histopathol. 1994. PMID: 7894150 Review.
-
Immunocytochemistry of myoepithelial cells in the salivary glands.Prog Histochem Cytochem. 2003;38(4):343-426. doi: 10.1016/s0079-6336(03)80001-3. Prog Histochem Cytochem. 2003. PMID: 14509196 Review.
Cited by
-
Concise Review: Salivary Gland Regeneration: Therapeutic Approaches from Stem Cells to Tissue Organoids.Stem Cells. 2017 Jan;35(1):97-105. doi: 10.1002/stem.2455. Epub 2016 Jul 15. Stem Cells. 2017. PMID: 27406006 Free PMC article. Review.
-
Salivary Gland Bioengineering.Bioengineering (Basel). 2023 Dec 26;11(1):28. doi: 10.3390/bioengineering11010028. Bioengineering (Basel). 2023. PMID: 38247905 Free PMC article. Review.
-
Stem Cell-Soluble Signals Enhance Multilumen Formation in SMG Cell Clusters.J Dent Res. 2015 Nov;94(11):1610-7. doi: 10.1177/0022034515600157. Epub 2015 Aug 18. J Dent Res. 2015. PMID: 26285810 Free PMC article.
-
Biomaterials-based strategies for salivary gland tissue regeneration.Biomater Sci. 2016 Apr;4(4):592-604. doi: 10.1039/c5bm00358j. Epub 2016 Feb 15. Biomater Sci. 2016. PMID: 26878077 Free PMC article.
-
Bioengineering in salivary gland regeneration.J Biomed Sci. 2022 Jun 6;29(1):35. doi: 10.1186/s12929-022-00819-w. J Biomed Sci. 2022. PMID: 35668440 Free PMC article. Review.
References
-
- Atala A.Recent developments in tissue engineering and regenerative medicine. Curr Opin Pediatr 18,167, 2006 - PubMed
-
- Atala A., Bauer S.B., Soker S., Yoo J.J., and Retik A.B.Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367,1241, 2006 - PubMed
-
- Daley W.P., Peters S.B., and Larsen M.Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 336,169, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

