Gelation chemistries for the encapsulation of nanoparticles in composite gel microparticles for lung imaging and drug delivery
- PMID: 24410445
- PMCID: PMC3981107
- DOI: 10.1021/bm4015232
Gelation chemistries for the encapsulation of nanoparticles in composite gel microparticles for lung imaging and drug delivery
Abstract
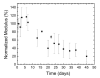
The formation of 10-40 μm composite gel microparticles (CGMPs) comprised of ∼100 nm drug containing nanoparticles (NPs) in a poly(ethylene glycol) (PEG) gel matrix is described. The CGMP particles enable targeting to the lung by filtration from the venous circulation. UV radical polymerization and Michael addition polymerization reactions are compared as approaches to form the PEG matrix. A fluorescent dye in the solid core of the NP was used to investigate the effect of reaction chemistry on the integrity of encapsulated species. When formed via UV radical polymerization, the fluorescence signal from the NPs indicated degradation of the encapsulated species by radical attack. The degradation decreased fluorescence by 90% over 15 min of UV exposure. When formed via Michael addition polymerization, the fluorescence was maintained. Emulsion processing using controlled shear stress enabled control of droplet size with narrow polydispersity. To allow for emulsion processing, the gelation rate was delayed by adjusting the solution pH. At a pH = 5.4, the gelation occurred at 3.5 h. The modulus of the gels was tuned over the range of 5 to 50 kPa by changing the polymer concentration between 20 and 70 vol %. NP aggregation during polymerization, driven by depletion forces, was controlled by the reaction kinetics. The ester bonds in the gel network enabled CGMP degradation. The gel modulus decreased by 50% over 27 days, followed by complete gel degradation after 55 days. This permits ultimate clearance of the CGMPs from the lungs. The demonstration of uniform delivery of 15.8 ± 2.6 μm CGMPs to the lungs of mice, with no deposition in other organs, is shown, and indicates the ability to concentrate therapeutics in the lung while avoiding off-target toxic exposure.
Figures

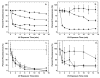
 ), IRG (
), IRG ( ), ACVA (
), ACVA ( ), and AMPA (
), and AMPA ( ). (For each point, n=3.) (b) GFP in sodium acetate buffer incubated with no initiator (
). (For each point, n=3.) (b) GFP in sodium acetate buffer incubated with no initiator ( ), IRG (
), IRG ( ), and ACVA (
), and ACVA ( ). (For each point, n=3.) (c) EtTP-5 nanoparticles in a solution of 25 vol% PEG-TA in sodium acetate buffer no initiator (
). (For each point, n=3.) (c) EtTP-5 nanoparticles in a solution of 25 vol% PEG-TA in sodium acetate buffer no initiator ( ), IRG (
), IRG ( ), and ACVA (
), and ACVA ( ). EtTP-5 nanoparticles in a solution of 25 vol% glycerol ethoxylate in sodium acetate buffer no initiator (
). EtTP-5 nanoparticles in a solution of 25 vol% glycerol ethoxylate in sodium acetate buffer no initiator ( ). (For each point, n>3.) (d) GFP in a solution of 25 vol% PEG-TA in sodium acetate buffer incubated with no initiator (
). (For each point, n>3.) (d) GFP in a solution of 25 vol% PEG-TA in sodium acetate buffer incubated with no initiator ( ), IRG (
), IRG ( ), and ACVA (
), and ACVA ( ). (For each point, n>3.)
). (For each point, n>3.)

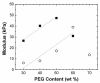
 ) and under fast gelling conditions in 1 mM triethylamine, pH 11.5, (
) and under fast gelling conditions in 1 mM triethylamine, pH 11.5, ( ) as a function of PEG triacrylate concentration. (For each formulation, n=1.)
) as a function of PEG triacrylate concentration. (For each formulation, n=1.)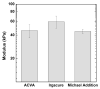


Similar articles
-
Controlled SLN Delivery by Thermoresponsive In-situ Forming Erodible Gels; A Whole-body and Organ Imaging Study.Curr Drug Deliv. 2018;15(4):510-519. doi: 10.2174/1567201815666180201093424. Curr Drug Deliv. 2018. PMID: 29422000
-
Biodistribution and renal clearance of biocompatible lung targeted poly(ethylene glycol) (PEG) nanogel aggregates.J Control Release. 2012 Nov 28;164(1):65-73. doi: 10.1016/j.jconrel.2012.09.011. Epub 2012 Oct 3. J Control Release. 2012. PMID: 23041417 Free PMC article.
-
Nanoparticle-Based Topical Ophthalmic Gel Formulation for Sustained Release of Hydrocortisone Butyrate.AAPS PharmSciTech. 2016 Apr;17(2):294-306. doi: 10.1208/s12249-015-0354-5. Epub 2015 Jun 18. AAPS PharmSciTech. 2016. PMID: 26085051 Free PMC article.
-
Nanoparticle-based topical ophthalmic formulation for sustained release of stereoisomeric dipeptide prodrugs of ganciclovir.Drug Deliv. 2016 Sep;23(7):2399-2409. doi: 10.3109/10717544.2014.996833. Epub 2015 Jan 7. Drug Deliv. 2016. PMID: 25564964
-
Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review.Adv Colloid Interface Sci. 2018 Mar;253:1-22. doi: 10.1016/j.cis.2018.02.002. Epub 2018 Feb 16. Adv Colloid Interface Sci. 2018. PMID: 29478671 Review.
Cited by
-
Single-Step Assembly of Multimodal Imaging Nanocarriers: MRI and Long-Wavelength Fluorescence Imaging.Adv Healthc Mater. 2015 Jun 24;4(9):1376-85. doi: 10.1002/adhm.201400766. Epub 2015 Apr 30. Adv Healthc Mater. 2015. PMID: 25925128 Free PMC article.
-
Polymeric nanoparticles in development for treatment of pulmonary infectious diseases.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016 Nov;8(6):842-871. doi: 10.1002/wnan.1401. Epub 2016 Mar 25. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016. PMID: 27016134 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

