Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications
- PMID: 24410814
- PMCID: PMC3898375
- DOI: 10.1186/1423-0127-21-3
Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications
Abstract
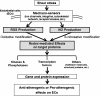
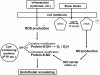
Hemodynamic shear stress, the blood flow-generated frictional force acting on the vascular endothelial cells, is essential for endothelial homeostasis under normal physiological conditions. Mechanosensors on endothelial cells detect shear stress and transduce it into biochemical signals to trigger vascular adaptive responses. Among the various shear-induced signaling molecules, reactive oxygen species (ROS) and nitric oxide (NO) have been implicated in vascular homeostasis and diseases. In this review, we explore the molecular, cellular, and vascular processes arising from shear-induced signaling (mechanotransduction) with emphasis on the roles of ROS and NO, and also discuss the mechanisms that may lead to excessive vascular remodeling and thus drive pathobiologic processes responsible for atherosclerosis. Current evidence suggests that NADPH oxidase is one of main cellular sources of ROS generation in endothelial cells under flow condition. Flow patterns and magnitude of shear determine the amount of ROS produced by endothelial cells, usually an irregular flow pattern (disturbed or oscillatory) producing higher levels of ROS than a regular flow pattern (steady or pulsatile). ROS production is closely linked to NO generation and elevated levels of ROS lead to low NO bioavailability, as is often observed in endothelial cells exposed to irregular flow. The low NO bioavailability is partly caused by the reaction of ROS with NO to form peroxynitrite, a key molecule which may initiate many pro-atherogenic events. This differential production of ROS and RNS (reactive nitrogen species) under various flow patterns and conditions modulates endothelial gene expression and thus results in differential vascular responses. Moreover, ROS/RNS are able to promote specific post-translational modifications in regulatory proteins (including S-glutathionylation, S-nitrosylation and tyrosine nitration), which constitute chemical signals that are relevant in cardiovascular pathophysiology. Overall, the dynamic interplay between local hemodynamic milieu and the resulting oxidative and S-nitrosative modification of regulatory proteins is important for ensuing vascular homeostasis. Based on available evidence, it is proposed that a regular flow pattern produces lower levels of ROS and higher NO bioavailability, creating an anti-atherogenic environment. On the other hand, an irregular flow pattern results in higher levels of ROS and yet lower NO bioavailability, thus triggering pro-atherogenic effects.
Figures





Similar articles
-
Anti-atherogenic effect of laminar shear stress via Nrf2 activation.Antioxid Redox Signal. 2011 Sep 1;15(5):1415-26. doi: 10.1089/ars.2010.3433. Epub 2011 Apr 21. Antioxid Redox Signal. 2011. PMID: 21126170 Review.
-
ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications.Free Radic Res. 2018 May;52(5):507-543. doi: 10.1080/10715762.2018.1457217. Epub 2018 Apr 19. Free Radic Res. 2018. PMID: 29589770 Review.
-
The role of glutathione and glutathione peroxidase in regulating cellular level of reactive oxygen and nitrogen species.Microvasc Res. 2020 Sep;131:104010. doi: 10.1016/j.mvr.2020.104010. Epub 2020 Apr 23. Microvasc Res. 2020. PMID: 32335268
-
Nitroxide antioxidant as a potential strategy to attenuate the oxidative/nitrosative stress induced by hydrogen peroxide plus nitric oxide in cultured neurons.Nitric Oxide. 2016 Apr 1;54:38-50. doi: 10.1016/j.niox.2016.02.001. Epub 2016 Feb 16. Nitric Oxide. 2016. PMID: 26891889
-
Redox signaling in hypertension.Cardiovasc Res. 2006 Jul 15;71(2):247-58. doi: 10.1016/j.cardiores.2006.05.001. Epub 2006 May 9. Cardiovasc Res. 2006. PMID: 16765337 Review.
Cited by
-
Reduction in MicroRNA-4488 Expression Induces NFκB Translocation in Venous Endothelial Cells Under Arterial Flow.Cardiovasc Drugs Ther. 2021 Feb;35(1):61-71. doi: 10.1007/s10557-020-06944-8. Cardiovasc Drugs Ther. 2021. PMID: 32902737
-
Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect?Curr Heart Fail Rep. 2015 Jun;12(3):215-22. doi: 10.1007/s11897-015-0254-8. Curr Heart Fail Rep. 2015. PMID: 25740404 Free PMC article. Review.
-
Role of Circular RNAs in Atherosclerosis through Regulation of Inflammation, Cell Proliferation, Migration, and Apoptosis: Focus on Atherosclerotic Cerebrovascular Disease.Medicina (Kaunas). 2023 Aug 14;59(8):1461. doi: 10.3390/medicina59081461. Medicina (Kaunas). 2023. PMID: 37629751 Free PMC article. Review.
-
Repair Effects of Astragalus Polysaccharides with Different Molecular Weights on Oxidatively Damaged HK-2 Cells.Sci Rep. 2019 Jul 8;9(1):9871. doi: 10.1038/s41598-019-46264-y. Sci Rep. 2019. PMID: 31285477 Free PMC article.
-
Mechanical force induces mitophagy-mediated anaerobic oxidation in periodontal ligament stem cells.Cell Mol Biol Lett. 2023 Jul 21;28(1):57. doi: 10.1186/s11658-023-00453-w. Cell Mol Biol Lett. 2023. PMID: 37480044 Free PMC article.
References
-
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

