FTO contributes to hepatic metabolism regulation through regulation of leptin action and STAT3 signalling in liver
- PMID: 24410832
- PMCID: PMC3896784
- DOI: 10.1186/1478-811X-12-4
FTO contributes to hepatic metabolism regulation through regulation of leptin action and STAT3 signalling in liver
Abstract
Background: The fat mass and obesity associated (FTO) gene is related to obesity and type 2 diabetes, but its function is still largely unknown. A link between leptin receptor-signal transducers and activators of transcription 3 (LepR-STAT3) signalling pathway and FTO was recently suggested in the hypothalamus. Because of the presence of FTO in liver and the role of LepR-STAT3 in the control of hepatic metabolism, we investigated both in vitro and in vivo the potential interrelationship between FTO and LepR-STAT3 signalling pathway in liver and the impact of FTO overexpression on leptin action and glucose homeostasis in liver of mice.
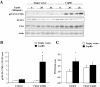
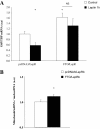
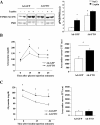
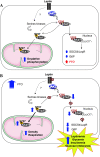
Results: We found that FTO protein expression is regulated by both leptin and IL-6, concomitantly to an induction of STAT3 tyrosine phosphorylation, in leptin receptor (LepRb) expressing HuH7 cells. In addition, FTO overexpression in vitro altered both leptin-induced Y705 and S727 STAT3 phosphorylation, leading to dysregulation of glucose-6-phosphatase (G6P) expression and mitochondrial density, respectively. In vivo, liver specific FTO overexpression in mice induced a reducetion of Y705 phosphorylation of STAT3 in nuclear fraction, associated with reduced SOCS3 and LepR mRNA levels and with an increased G6P expression. Interestingly, FTO overexpression also induced S727 STAT3 phosphorylation in liver mitochondria, resulting in an increase of mitochondria function and density. Altogether, these data indicate that FTO promotes mitochondrial recruitment of STAT3 to the detriment of its nuclear localization, affecting in turn oxidative metabolism and the expression of leptin-targeted genes. Interestingly, these effects were associated in mice with alterations of leptin action and hyperleptinemia, as well as hyperglycemia, hyperinsulinemia and glucose intolerance.
Conclusions: Altogether, these data point a novel regulatory loop between FTO and leptin-STAT3 signalling pathways in liver cells, and highlight a new role of FTO in the regulation of hepatic leptin action and glucose metabolism.
Figures




Similar articles
-
Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling.J Biol Chem. 2011 Jan 21;286(3):2155-70. doi: 10.1074/jbc.M110.188482. Epub 2010 Oct 31. J Biol Chem. 2011. PMID: 21037323 Free PMC article.
-
Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction.Mol Med. 2011 May-Jun;17(5-6):523-32. doi: 10.2119/molmed.2010.00134. Epub 2011 Jan 21. Mol Med. 2011. PMID: 21267512 Free PMC article.
-
Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity.PLoS One. 2012;7(1):e30485. doi: 10.1371/journal.pone.0030485. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276206 Free PMC article.
-
Leptin receptor signaling and the regulation of mammalian physiology.Int J Obes (Lond). 2008 Dec;32 Suppl 7(Suppl 7):S8-12. doi: 10.1038/ijo.2008.232. Int J Obes (Lond). 2008. PMID: 19136996 Free PMC article. Review.
-
FTO and obesity: mechanisms of association.Curr Diab Rep. 2014;14(5):486. doi: 10.1007/s11892-014-0486-0. Curr Diab Rep. 2014. PMID: 24627050 Review.
Cited by
-
Genome-wide association study of endo-parasite phenotypes using imputed whole-genome sequence data in dairy and beef cattle.Genet Sel Evol. 2019 Apr 18;51(1):15. doi: 10.1186/s12711-019-0457-7. Genet Sel Evol. 2019. PMID: 30999842 Free PMC article.
-
The 'Fat Mass and Obesity Related' (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance.Curr Obes Rep. 2015 Mar;4(1):73-91. doi: 10.1007/s13679-015-0143-1. Curr Obes Rep. 2015. PMID: 26627093 Review.
-
Lactylation-driven FTO targets CDK2 to aggravate microvascular anomalies in diabetic retinopathy.EMBO Mol Med. 2024 Feb;16(2):294-318. doi: 10.1038/s44321-024-00025-1. Epub 2024 Jan 31. EMBO Mol Med. 2024. PMID: 38297099 Free PMC article.
-
Emerging Roles of FTO in Neuropsychiatric Disorders.Biomed Res Int. 2022 Apr 26;2022:2677312. doi: 10.1155/2022/2677312. eCollection 2022. Biomed Res Int. 2022. PMID: 35528183 Free PMC article. Review.
-
The role of m6A modification in physiology and disease.Cell Death Dis. 2020 Nov 8;11(11):960. doi: 10.1038/s41419-020-03143-z. Cell Death Dis. 2020. PMID: 33162550 Free PMC article. Review.
References
-
- Bravard A, Lefai E, Meugnier E, Pesenti S, Disse E, Vouillarmet J, Peretti N, Rabasa-Lhoret R, Laville M, Vidal H, Rieusset J. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes. 2011;60:258–268. doi: 10.2337/db10-0281. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

