Evolutionary origins of sensation in metazoans: functional evidence for a new sensory organ in sponges
- PMID: 24410880
- PMCID: PMC3890488
- DOI: 10.1186/1471-2148-14-3
Evolutionary origins of sensation in metazoans: functional evidence for a new sensory organ in sponges
Abstract
Background: One of the hallmarks of multicellular organisms is the ability of their cells to trigger responses to the environment in a coordinated manner. In recent years primary cilia have been shown to be present as 'antennae' on almost all animal cells, and are involved in cell-to-cell signaling in development and tissue homeostasis; how this sophisticated sensory system arose has been little-studied and its evolution is key to understanding how sensation arose in the Animal Kingdom. Sponges (Porifera), one of the earliest evolving phyla, lack conventional muscles and nerves and yet sense and respond to changes in their fluid environment. Here we demonstrate the presence of non-motile cilia in sponges and studied their role as flow sensors.
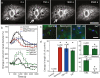
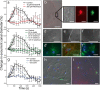
Results: Demosponges excrete wastes from their body with a stereotypic series of whole-body contractions using a structure called the osculum to regulate the water-flow through the body. In this study we show that short cilia line the inner epithelium of the sponge osculum. Ultrastructure of the cilia shows an absence of a central pair of microtubules and high speed imaging shows they are non-motile, suggesting they are not involved in generating flow. In other animals non-motile, 'primary', cilia are involved in sensation. Here we show that molecules known to block cationic ion channels in primary cilia and which inhibit sensory function in other organisms reduce or eliminate sponge contractions. Removal of the cilia using chloral hydrate, or removal of the whole osculum, also stops the contractions; in all instances the effect is reversible, suggesting that the cilia are involved in sensation. An analysis of sponge transcriptomes shows the presence of several transient receptor potential (TRP) channels including PKD channels known to be involved in sensing changes in flow in other animals. Together these data suggest that cilia in sponge oscula are involved in flow sensation and coordination of simple behaviour.
Conclusions: This is the first evidence of arrays of non-motile cilia in sponge oscula. Our findings provide support for the hypothesis that the cilia are sensory, and if true, the osculum may be considered a sensory organ that is used to coordinate whole animal responses in sponges. Arrays of primary cilia like these could represent the first step in the evolution of sensory and coordination systems in metazoans.
Figures



Similar articles
-
The analysis of eight transcriptomes from all poriferan classes reveals surprising genetic complexity in sponges.Mol Biol Evol. 2014 May;31(5):1102-20. doi: 10.1093/molbev/msu057. Epub 2014 Feb 4. Mol Biol Evol. 2014. PMID: 24497032
-
Think like a sponge: The genetic signal of sensory cells in sponges.Dev Biol. 2017 Nov 1;431(1):93-100. doi: 10.1016/j.ydbio.2017.06.012. Epub 2017 Jun 21. Dev Biol. 2017. PMID: 28647138 Review.
-
Wnt signaling and polarity in freshwater sponges.BMC Evol Biol. 2018 Feb 2;18(1):12. doi: 10.1186/s12862-018-1118-0. BMC Evol Biol. 2018. PMID: 29394881 Free PMC article.
-
Origin of the neuro-sensory system: new and expected insights from sponges.Integr Zool. 2009 Sep;4(3):294-308. doi: 10.1111/j.1749-4877.2009.00167.x. Integr Zool. 2009. PMID: 21392302 Review.
-
Effects of Seasonal Anoxia on the Microbial Community Structure in Demosponges in a Marine Lake in Lough Hyne, Ireland.mSphere. 2021 Feb 3;6(1):e00991-20. doi: 10.1128/mSphere.00991-20. mSphere. 2021. PMID: 33536324 Free PMC article.
Cited by
-
Spiculous skeleton formation in the freshwater sponge Ephydatia fluviatilis under hypergravity conditions.PeerJ. 2019 Jan 4;6:e6055. doi: 10.7717/peerj.6055. eCollection 2019. PeerJ. 2019. PMID: 30631642 Free PMC article.
-
The brain: a concept in flux.Philos Trans R Soc Lond B Biol Sci. 2019 Jun 10;374(1774):20180383. doi: 10.1098/rstb.2018.0383. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31006364 Free PMC article. Review.
-
Gastric pouches and the mucociliary sole: setting the stage for nervous system evolution.Philos Trans R Soc Lond B Biol Sci. 2015 Dec 19;370(1684):20150286. doi: 10.1098/rstb.2015.0286. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26554050 Free PMC article. Review.
-
ATP and glutamate coordinate contractions in the freshwater sponge Ephydatia muelleri.J Exp Biol. 2025 Feb 1;228(3):JEB248010. doi: 10.1242/jeb.248010. Epub 2025 Feb 12. J Exp Biol. 2025. PMID: 39936310 Free PMC article.
-
Environmental complexity and regularity shape the evolution of cognition.Proc Biol Sci. 2024 Oct;291(2033):20241524. doi: 10.1098/rspb.2024.1524. Epub 2024 Oct 23. Proc Biol Sci. 2024. PMID: 39437844 Free PMC article.
References
-
- Ryan TJ, Grant SGN. The origin and evolution of synapses. Nat Rev Neurosci. 2009;10:701–712. - PubMed
-
- Fujiu K, Nakayama Y, Iida H, Sokabe M, Yoshimura K. Mechanoreception in motile flagella of Chlamydomonas. Nat Cell Biol. 2011;13:630–632. - PubMed
-
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. - PubMed
-
- Meech RW. Non-neural reflexes: sponges and the origins of behaviour. Curr Biol. 2008;18:R70–R72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

