Exploiting tumor cell senescence in anticancer therapy
- PMID: 24411464
- PMCID: PMC4163898
- DOI: 10.5483/bmbrep.2014.47.2.005
Exploiting tumor cell senescence in anticancer therapy
Abstract
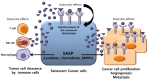
Cellular senescence is a physiological process of irreversible cell-cycle arrest that contributes to various physiological and pathological processes of aging. Whereas replicative senescence is associated with telomere attrition after repeated cell division, stress-induced premature senescence occurs in response to aberrant oncogenic signaling, oxidative stress, and DNA damage which is independent of telomere dysfunction. Recent evidence indicates that cellular senescence provides a barrier to tumorigenesis and is a determinant of the outcome of cancer treatment. However, the senescence-associated secretory phenotype, which contributes to multiple facets of senescent cancer cells, may influence both cancer-inhibitory and cancer-promoting mechanisms of neighboring cells. Conventional treatments, such as chemo- and radiotherapies, preferentially induce premature senescence instead of apoptosis in the appropriate cellular context. In addition, treatment-induced premature senescence could compensate for resistance to apoptosis via alternative signaling pathways. Therefore, we believe that an intensive effort to understand cancer cell senescence could facilitate the development of novel therapeutic strategies for improving the efficacy of anticancer therapies. This review summarizes the current understanding of molecular mechanisms, functions, and clinical applications of cellular senescence for anticancer therapy.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

