Acquired tumor cell radiation resistance at the treatment site is mediated through radiation-orchestrated intercellular communication
- PMID: 24411622
- PMCID: PMC4034458
- DOI: 10.1016/j.ijrobp.2013.11.215
Acquired tumor cell radiation resistance at the treatment site is mediated through radiation-orchestrated intercellular communication
Abstract
Purpose: Radiation resistance induced in cancer cells that survive after radiation therapy (RT) could be associated with increased radiation protection, limiting the therapeutic benefit of radiation. Herein we investigated the sequential mechanistic molecular orchestration involved in radiation-induced radiation protection in tumor cells.
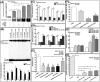
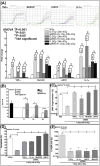
Results: Radiation, both in the low-dose irradiation (LDIR) range (10, 50, or 100 cGy) or at a higher, challenge dose IR (CDIR), 4 Gy, induced dose-dependent and sustained NFκB-DNA binding activity. However, a robust and consistent increase was seen in CDIR-induced NFκB activity, decreased DNA fragmentation, apoptosis, and cytotoxicity and attenuation of CDIR-inhibited clonal expansion when the cells were primed with LDIR prior to challenge dose. Furthermore, NFκB manipulation studies with small interfering RNA (siRNA) silencing or p50/p65 overexpression unveiled the influence of LDIR-activated NFκB in regulating CDIR-induced DNA fragmentation and apoptosis. LDIR significantly increased the transactivation/translation of the radiation-responsive factors tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), cMYC, and SOD2. Coculture experiments exhibit LDIR-influenced radiation protection and increases in cellular expression, secretion, and activation of radiation-responsive molecules in bystander cells. Individual gene-silencing approach with siRNAs coupled with coculture studies showed the influence of LDIR-modulated TNF-α, IL-1α, cMYC, and SOD2 in induced radiation protection in bystander cells. NFκB inhibition/overexpression studies coupled with coculture experiments demonstrated that TNF-α, IL-1α, cMYC, and SOD2 are selectively regulated by LDIR-induced NFκB.
Conclusions: Together, these data strongly suggest that scattered LDIR-induced NFκB-dependent TNF-α, IL-1α, cMYC, and SOD2 mediate radiation protection to the subsequent challenge dose in tumor cells.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: none.
Figures


References
-
- American Cancer Society. Cancer facts and figures 2012. Atlanta: American Cancer Society; 2012.
-
- Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician. 2010;82:381–388. - PubMed
-
- Russell J, Wheldon TE, Stanton P. A radioresistant variant derived from a human neuroblastoma cell line is less prone to radiation-induced apoptosis. Cancer Res. 1995;55:4915–4921. - PubMed
-
- Stecca C, Gerber GB. Adaptive response to DNA-damaging agents: A review of potential mechanisms. Biochem Pharmacol. 1998;55:941–951. - PubMed
-
- Madhusoodhanan R, Natarajan M, Veeraraghavan J, et al. NFκB activity and transcriptional responses in human breast adenocarcinoma cells after single and fractionated irradiation. Cancer Biol Ther. 2009;8:765–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

