Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration
- PMID: 24411675
- PMCID: PMC4215726
- DOI: 10.1016/j.biomaterials.2013.11.078
Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration
Abstract
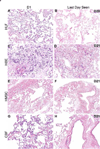
Acellular scaffolds from complex whole organs such as lung are being increasingly studied for ex vivo organ generation and for in vitro studies of cell-extracellular matrix interactions. We have established effective methods for efficient de and recellularization of large animal and human lungs including techniques which allow multiple small segments (∼ 1-3 cm(3)) to be excised that retain 3-dimensional lung structure. Coupled with the use of a synthetic pleural coating, cells can be selectively physiologically inoculated via preserved vascular and airway conduits. Inoculated segments can be further sliced for high throughput studies. Further, we demonstrate thermography as a powerful noninvasive technique for monitoring perfusion decellularization and for evaluating preservation of vascular and airway networks following human and porcine lung decellularization. Collectively, these techniques are a significant step forward as they allow high throughput in vitro studies from a single lung or lobe in a more biologically relevant, three-dimensional acellular scaffold.
Keywords: Acellular matrix; Endothelial cell; Epithelial cell; Extracellular matrix (ECM); Human lung fibroblast; Mesenchymal stem cell.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures















References
-
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. - PubMed
-
- Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–2580. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

