Lupus nephritis: the evolving role of novel therapeutics
- PMID: 24411715
- PMCID: PMC4159074
- DOI: 10.1053/j.ajkd.2013.11.023
Lupus nephritis: the evolving role of novel therapeutics
Abstract
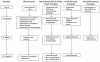
Immune complex accumulation in the kidney is the hallmark of lupus nephritis and triggers a series of events that result in kidney inflammation and injury. Cytotoxic agents and corticosteroids are standard of care for lupus nephritis treatment, but are associated with considerable morbidity and suboptimal outcomes. Recently, there has been interest in using novel biologic agents and small molecules to treat lupus nephritis. These therapies can be broadly categorized as anti-inflammatory (laquinamod, anti-tumor necrosis factor-like weak inducer of apotosis, anti-C5, and retinoids), antiautoimmunity (anti-CD20, anti-interferon α, and costimulatory blockers), or both (anti-interleukin 6 and proteasome inhibitors). Recent lupus nephritis clinical trials applied biologics or small molecules of any category to induction treatment, seeking short-term end points of complete renal response. These trials in general have not succeeded. When lupus nephritis comes to clinical attention during the inflammatory stage of the disease, the autoimmune stage leading to kidney inflammation will have been active for some time. The optimal approach for using novel therapies may be to initially target kidney inflammation to preserve renal parenchyma, followed by suppression of autoimmunity. In this review, we discuss novel lupus nephritis therapies and how they fit into a combinatorial treatment strategy based on the pathogenic stage.
Keywords: Lupus nephritis; biologics; novel therapies; small molecules; systemic lupus erythematosus (SLE).
Published by Elsevier Inc.
Figures

References
-
- Austin HA, Klippel JH, Balow JE, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614–619. - PubMed
-
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–2131. - PubMed
-
- Sanz AB, Sanchez-Nino MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80(7):708–718. - PubMed
-
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose versus high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69:61–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

