Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice
- PMID: 24411733
- PMCID: PMC3894588
- DOI: 10.1016/j.neuron.2013.10.052
Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice
Erratum in
- Neuron. 2014 Jun 4;82(5):1186-7
Abstract
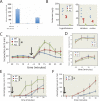
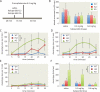
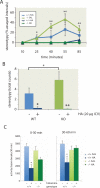
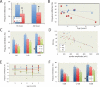
Tourette syndrome (TS) is characterized by tics, sensorimotor gating deficiencies, and abnormalities of cortico-basal ganglia circuits. A mutation in histidine decarboxylase (Hdc), the key enzyme for the biosynthesis of histamine (HA), has been implicated as a rare genetic cause. Hdc knockout mice exhibited potentiated tic-like stereotypies, recapitulating core phenomenology of TS; these were mitigated by the dopamine (DA) D2 antagonist haloperidol, a proven pharmacotherapy, and by HA infusion into the brain. Prepulse inhibition was impaired in both mice and humans carrying Hdc mutations. HA infusion reduced striatal DA levels; in Hdc knockout mice, striatal DA was increased and the DA-regulated immediate early gene Fos was upregulated. DA D2/D3 receptor binding was altered both in mice and in humans carrying the Hdc mutation. These data confirm histidine decarboxylase deficiency as a rare cause of TS and identify HA-DA interactions in the basal ganglia as an important locus of pathology.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Acevedo SF, Ohtsu H, Benice TS, Rizk-Jackson A, Raber J. Age-dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc−/−) mice. Brain Res. 2006;1071:113–123. - PubMed
-
- Albin RL. Neurobiology of basal ganglia and Tourette syndrome: striatal and dopamine function. Advances in neurology. 2006;99:99–106. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–375. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9:357–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR024139/RR/NCRR NIH HHS/United States
- 2P50AA012870/AA/NIAAA NIH HHS/United States
- R01MH091861/MH/NIMH NIH HHS/United States
- R01 MH091861/MH/NIMH NIH HHS/United States
- T32MH014276/MH/NIMH NIH HHS/United States
- R25 MH077823/MH/NIMH NIH HHS/United States
- T32MH018268/MH/NIMH NIH HHS/United States
- PL1 DA024860/DA/NIDA NIH HHS/United States
- T32 MH018268/MH/NIMH NIH HHS/United States
- UL1RR024139/RR/NCRR NIH HHS/United States
- R01NS056276/NS/NINDS NIH HHS/United States
- D43TW06166/TW/FIC NIH HHS/United States
- K08MH081190/MH/NIMH NIH HHS/United States
- D43 TW006166/TW/FIC NIH HHS/United States
- K08 MH081190/MH/NIMH NIH HHS/United States
- R01 NS056276/NS/NINDS NIH HHS/United States
- T32 MH014276/MH/NIMH NIH HHS/United States
- P50 AA012870/AA/NIAAA NIH HHS/United States
- PL1DA024860/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
