Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease
- PMID: 24411735
- PMCID: PMC3950970
- DOI: 10.1016/j.neuron.2013.10.051
Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease
Abstract
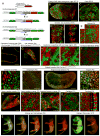
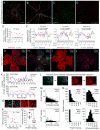
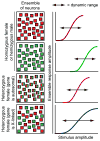
Female eutherian mammals use X chromosome inactivation (XCI) to epigenetically regulate gene expression from ∼4% of the genome. To quantitatively map the topography of XCI for defined cell types at single cell resolution, we have generated female mice that carry X-linked, Cre-activated, and nuclear-localized fluorescent reporters--GFP on one X chromosome and tdTomato on the other. Using these reporters in combination with different Cre drivers, we have defined the topographies of XCI mosaicism for multiple CNS cell types and of retinal vascular dysfunction in a model of Norrie disease. Depending on cell type, fluctuations in the XCI mosaic are observed over a wide range of spatial scales, from neighboring cells to left versus right sides of the body. These data imply a major role for XCI in generating female-specific, genetically directed, stochastic diversity in eutherian mammals on spatial scales that would be predicted to affect CNS function within and between individuals.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Berger W, Ropers HH. Norrie Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw Hill; 2001. pp. 5977–5985.
-
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. - PubMed
-
- Cattanach BM. Control of chromosome inactivation. Annu Rev Genet. 1975;9:1–18. - PubMed
-
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

