Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry
- PMID: 24411738
- PMCID: PMC3899717
- DOI: 10.1016/j.neuron.2013.10.041
Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry
Abstract
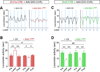
Structural plasticity in the adult brain is essential for adaptive behavior. We have found a remarkable anatomical plasticity in the basal ganglia of adult mice that is regulated by dopamine D2 receptors (D2Rs). By modulating neuronal excitability, striatal D2Rs bidirectionally control the density of direct pathway collaterals in the globus pallidus that bridge the direct pathway with the functionally opposing indirect pathway. An increase in bridging collaterals is associated with enhanced inhibition of pallidal neurons in vivo and disrupted locomotor activation after optogenetic stimulation of the direct pathway. Chronic blockade with haloperidol, an antipsychotic medication used to treat schizophrenia, decreases the extent of bridging collaterals and rescues the locomotor imbalance. These findings identify a role for bridging collaterals in regulating the concerted balance of striatal output and may have important implications for understanding schizophrenia, a disease involving excessive activation of striatal D2Rs that is treated with D2R blockers.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. - PubMed
-
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

