β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors
- PMID: 24411942
- PMCID: PMC4017355
- DOI: 10.1016/j.cmet.2013.12.003
β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors
Abstract
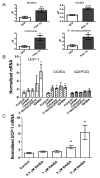
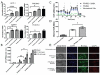
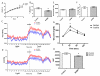
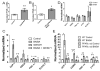
The transcriptional coactivator peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) regulates metabolic genes in skeletal muscle and contributes to the response of muscle to exercise. Muscle PGC-1α transgenic expression and exercise both increase the expression of thermogenic genes within white adipose. How the PGC-1α-mediated response to exercise in muscle conveys signals to other tissues remains incompletely defined. We employed a metabolomic approach to examine metabolites secreted from myocytes with forced expression of PGC-1α, and identified β-aminoisobutyric acid (BAIBA) as a small molecule myokine. BAIBA increases the expression of brown adipocyte-specific genes in white adipocytes and β-oxidation in hepatocytes both in vitro and in vivo through a PPARα-mediated mechanism, induces a brown adipose-like phenotype in human pluripotent stem cells, and improves glucose homeostasis in mice. In humans, plasma BAIBA concentrations are increased with exercise and inversely associated with metabolic risk factors. BAIBA may thus contribute to exercise-induced protection from metabolic diseases.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Come on BAIBA light my fire.Cell Metab. 2014 Jan 7;19(1):1-2. doi: 10.1016/j.cmet.2013.12.007. Cell Metab. 2014. PMID: 24411934
-
Metabolism: Feel the burn--muscle metabolite couples exercise to heat production.Nat Rev Endocrinol. 2014 Apr;10(4):188. doi: 10.1038/nrendo.2014.4. Epub 2014 Jan 28. Nat Rev Endocrinol. 2014. PMID: 24468648 No abstract available.
References
-
- al Yacoub N, Romanowska M, Haritonova N, Foerster J. Optimized production and concentration of lentiviral vectors containing large inserts. J Gene Med. 2007;9:579–584. - PubMed
-
- Aoi W, Naito Y, Hang LP, Uchiyama K, Akagiri S, Mizushima K, Yoshikawa T. Regular exercise prevents high-sucrose diet-induced fatty liver via improvement of hepatic lipid metabolism. Biochem Biophys Res Commun. 2011;413:330–335. - PubMed
-
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. - PubMed
-
- Begriche K, Massart J, Abbey-Toby A, Igoudjil A, Letteron P, Fromenty B. Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity (Silver Spring) 2008;16:2053–2067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

