Critical role for mast cell Stat5 activity in skin inflammation
- PMID: 24412367
- PMCID: PMC4329986
- DOI: 10.1016/j.celrep.2013.12.029
Critical role for mast cell Stat5 activity in skin inflammation
Abstract
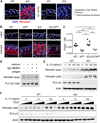
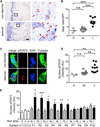
Atopic dermatitis (AD) is a chronic inflammatory skin disease. Here, we show that phospholipase C-β3 (PLC-β3)-deficient mice spontaneously develop AD-like skin lesions and more severe allergen-induced dermatitis than wild-type mice. Mast cells were required for both AD models and remarkably increased in the skin of Plcb3(-/-) mice because of the increased Stat5 and reduced SHP-1 activities. Mast cell-specific deletion of Stat5 gene ameliorated allergen-induced dermatitis, whereas that of Shp1 gene encoding Stat5-inactivating SHP-1 exacerbated it. PLC-β3 regulates the expression of periostin in fibroblasts and TSLP in keratinocytes, two proteins critically involved in AD pathogenesis. Furthermore, polymorphisms in PLCB3, SHP1, STAT5A, and STAT5B genes were associated with human AD. Mast cell expression of PLC-β3 was inversely correlated with that of phospho-STAT5, and increased mast cells with high levels of phospho-STAT5 were found in lesional skin of some AD patients. Therefore, STAT5 regulatory mechanisms in mast cells are important for AD pathogenesis.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



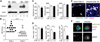

References
-
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007;204:253–258. - PMC - PubMed
-
- Bieber T. Atopic dermatitis. N. Engl. J. Med. 2008;358:1483–1494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

