Ankyrin-G directly binds to kinesin-1 to transport voltage-gated Na+ channels into axons
- PMID: 24412576
- PMCID: PMC3970081
- DOI: 10.1016/j.devcel.2013.11.023
Ankyrin-G directly binds to kinesin-1 to transport voltage-gated Na+ channels into axons
Abstract
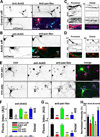
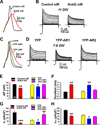
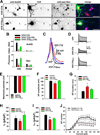
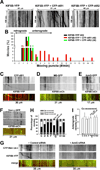
Action potentials (APs) propagating along axons require the activation of voltage-gated Na(+) (Nav) channels. How Nav channels are transported into axons is unknown. We show that KIF5/kinesin-1 directly binds to ankyrin-G (AnkG) to transport Nav channels into axons. KIF5 and Nav1.2 channels bind to multiple sites in the AnkG N-terminal domain that contains 24 ankyrin repeats. Disrupting AnkG-KIF5 binding with small interfering RNA or dominant-negative constructs markedly reduced Nav channel levels at the axon initial segment (AIS) and along entire axons, thereby decreasing AP firing. Live-cell imaging showed that fluorescently tagged AnkG or Nav1.2 cotransported with KIF5 along axons. Deleting AnkG in vivo or virus-mediated expression of a dominant-negative KIF5 construct specifically decreased the axonal level of Nav, but not Kv1.2, channels in mouse cerebellum. These results indicate that AnkG functions as an adaptor to link Nav channels to KIF5 during axonal transport before anchoring them to the AIS and nodes of Ranvier.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

